Ocular Manifestations of Acquired Immunodeficiency Syndrome
Article information
Abstract
Purpose
To investigate the patterns and risk factors of the ocular manifestations of acquired immunodeficiency syndrome (AIDS) and their correlation with CD4+ count in the era of highly active antiretroviral therapy (HAART).
Methods
This retrospective study examined 127 AIDS patients who presented to Soonchunhyang University Hospital. Data were collected from patient interviews, clinical examinations, and laboratory investigations. Ophthalmologic examinations included the best-corrected visual acuity, intraocular pressure, anterior segment and adnexal examination, and dilated fundus examination.
Results
Of the 127 patients with AIDS, 118 were on HAART and 9 were not. The mean CD4+ count was 266.7 ± 209.1 cells/µL. There were ocular manifestations in 61 patients (48.0%). The incidence of anterior segment manifestations was higher than posterior segment manifestations at 28.3% and 19.7%, respectively. The mean CD4+ count was significantly (p < 0.05) lower in the patients with posterior versus anterior segment ocular manifestations. The most common ocular manifestation was retinal microvasculopathy (15.0%), followed by keratoconjunctivitis sicca (14.2%), conjunctival microvasculopathy (9.4%), cytomegalovirus retinitis (3.1%), herpes zoster ophthalmicus (2.4%), and blepharitis (1.6%). Retinal microvasculopathy and cytomegalovirus retinitis were common in patients with CD4+ counts <200 cells/µL, while keratoconjunctivitis sicca and conjunctival microvasculopathy were common in patients with CD4+ counts of 200 to 499 cells/µL. There was a significant (p < 0.05) association between ocular manifestation and CD4+ count or age.
Conclusions
The introduction of HAART has changed the landscape of ocular presentations in patients with AIDS. In this study, anterior segment and external ocular manifestations occurred more frequently than posterior segment manifestations. Also, the mean CD4+ count was significantly lower in patients with posterior segment ocular manifestations versus anterior segment ocular manifestations. We found that CD4+ count and age >35 years were independent risk factors for developing ocular manifestations.
Since the human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) epidemic began in 1981 [1], ocular involvement has been a common finding [234], and loss of vision is a serious problem in people living with HIV [5]. Before the era of highly active antiretroviral therapy (HAART), ocular involvement was estimated to develop in 50% to 75% of patients with HIV infection [67], including retinal microvasculopathy, vascular occlusions, opportunistic infections such as cytomegalovirus (CMV) retinitis, and progressive outer retinal necrosis [8]. A variety of other important AIDS-related manifestations involve the anterior segment, ocular surface, and adnexae [9].
The success of HAART in restoring CD4+ T-cell immunity has significantly affected the pattern and natural history of ocular manifestations of HIV infection [10]. The overall incidence of ocular manifestations of HIV infection in people living with HIV/AIDS has decreased to less than 40%. In the HAART era, there has been an estimated 80% decrease in the incidence of CMV retinitis [11]. With HAART, there have been improvements in the length [12] and quality of life of individuals with HIV. The incidence of sight-threatening complications has decreased, but attention should be still paid to other minor problems requiring ophthalmic care, including anterior segment and external ocular manifestations, which can affect the quality of life [2].
Therefore, this study describes the patterns and risk factors of the ocular manifestations of AIDS in the HAART era and their correlation to the CD4+ count.
Materials and Methods
A retrospective chart review was performed on patients seen at Soonchunhyang University Hospital from August 2004 to April 2014. Of 417 total HIV/AIDS patients, 127 were referred to the department of ophthalmology for an evaluation of ocular manifestations. Of these 127 patients, 118 were on HAART and 9 were not. Patients with additional medical problems such as diabetes mellitus, hyper-tension, and ocular trauma, which can have manifestations overlapping with AIDS, were excluded from the study.
Data were collected from patient interviews, clinical examinations, and laboratory investigations. Ophthalmologic examinations included the best-corrected visual acuity, in-traocular pressure, anterior segment and adnexal examination, and dilated fundus examination.
Keratoconjunctivitis sicca was diagnosed using the Ocular Surface Disease Index (OSDI) questionnaire, which includes the following three domains: ocular symptoms, vision-related function, and environmental triggers [13]. Based on their OSDI scores, patients were categorized into two groups: those with a normal ocular surface (0 to 12 points) or those with ocular surface disease (≥13 points) [14]. Conjunctival microvasculopathy was diagnosed when patients presented with microaneurysms, segmental dilation of the venules, and adjacent narrowing of the arterioles, particularly with involvement of the inferior perilimbal bulbar conjunctiva [15].
Retinal microvasculopathy was diagnosed based on the following clinical manifestations: cotton-wool spots, microaneurysms, retinal hemorrhages, telangiectatic vascular changes, and the presence of capillary non-perfusion [16]. The diagnosis of CMV retinitis was based on clinical signs and symptoms [17181920]. CMV's characteristic retinal lesion has a dry appearing granular border surrounding an area of retinal edema and full-thickness retinal necrosis (Fig. 1).
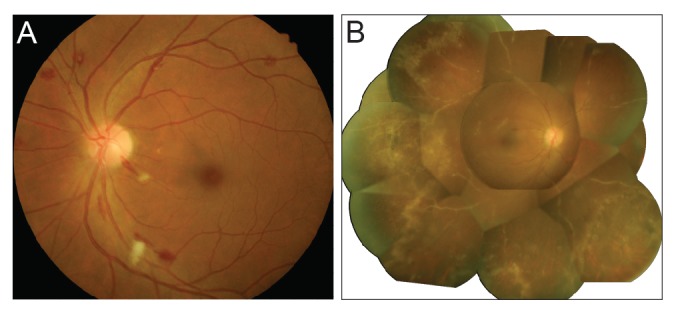
(A) Fundus photograph showing retinal microvasculopathy with numerous cotton-wool spots and retinal hemorrhages. (B) Fundus photograph showing characteristic findings of cytomegalovirus retinitis, including prominent perivascular sheating and retinal necrosis with irregular granular borders.
The data were analyzed using IBM SPSS ver. 21 (IBM Corp., Armonk, NY, USA). Chi-square tests were used and p < 0.05 was considered significant. Student's t-tests, Fisher's exact tests, and multiple logistic regression were used to examine the associations and differences between the variables.
The study followed the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of Soonchunhyang University.
Results
Of the 417 subjects with AIDS, 127 patients were assessed. The majority of patients (96.1%) were male. The mean age of the patients was 38.7 (range, 19 to 76) years, and 36.9% of the patients were 30 to 39 years old. Of the 127 patients being assessed, 118 were on HAART and 9 were not. The mean CD4+ count was 266.7 ± 209.1 cells/µL. The route of HIV infection was documented in 56 patients (44.1%) with the most common route being homo-sexual contact in 40 patients (71.4%), followed by heterosexual contact in 15 (26.8%). One patient was suspected to have been infected via blood transfusion.
Of the 127 AIDS patients, 61 (48.0%) had ocular manifestations. The incidence of ocular manifestation in males (47.5%) was lower than in females (60.0%), but this difference was not significant (p = 0.585). The mean age of the patients with and without ocular manifestation was 40.1 ± 10.8 and 37.3 ± 12.6 years, respectively (p = 0.172). Ocular manifestations were most common in the 30 to 39 years age group (36.9%), followed by 40 to 49 years (24.6%), and 20 to 29 years (20.0%). However, the differences in the dis-tribution of ocular manifestation between the age groups were not significant (p = 0.240). Patients with ocular manifestations had lower CD4+ counts than patients without ocular manifestations (233.4 vs. 297.4 cells/µL, respectively), but this difference was not significant (p = 0.084) (Table 1).
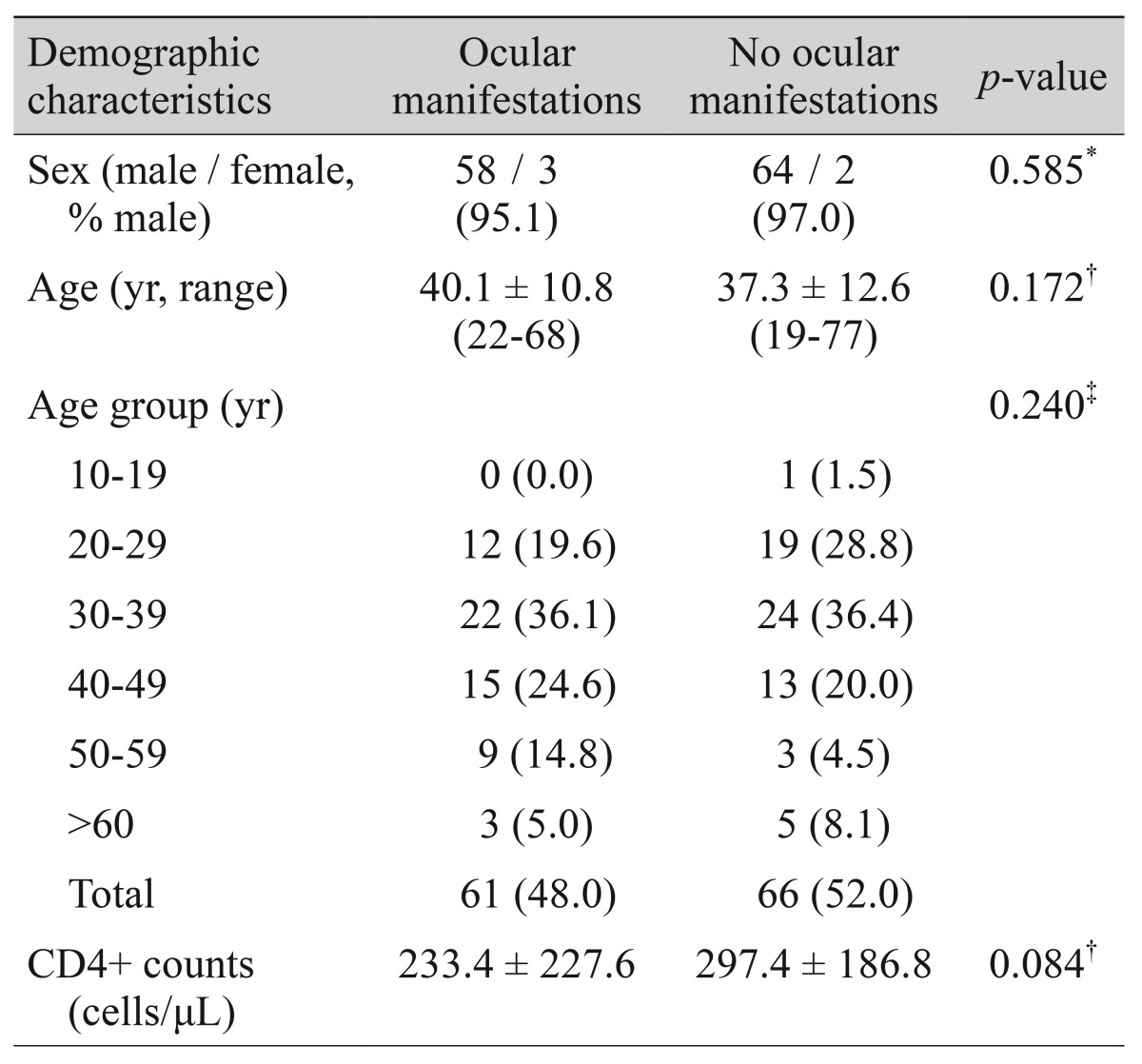
Demographic characteristics and ocular manifestations of human immunodeficiency virus-infected or acquired immunodeficiency syndrome patients
The incidence of anterior segment manifestations was higher than posterior segment manifestations, at 28.3% and 19.7%, respectively. The mean ± SD CD4+ count for those patients with anterior and posterior segment ocular manifestations was significantly different at 335.9 ± 240.8 and 85.7 ± 80.6, respectively (p < 0.05). Relative frequencies of ocular manifestations differed in patients grouped according to the CD4+ counts. Specifically, anterior segment manifestations were common in patients with CD4+ counts of 200 to 499 cells/µL (58.3%), while posterior segment manifestations were common in patients with CD4+ counts <200 cells/µL (88.0%) (Fig. 2).
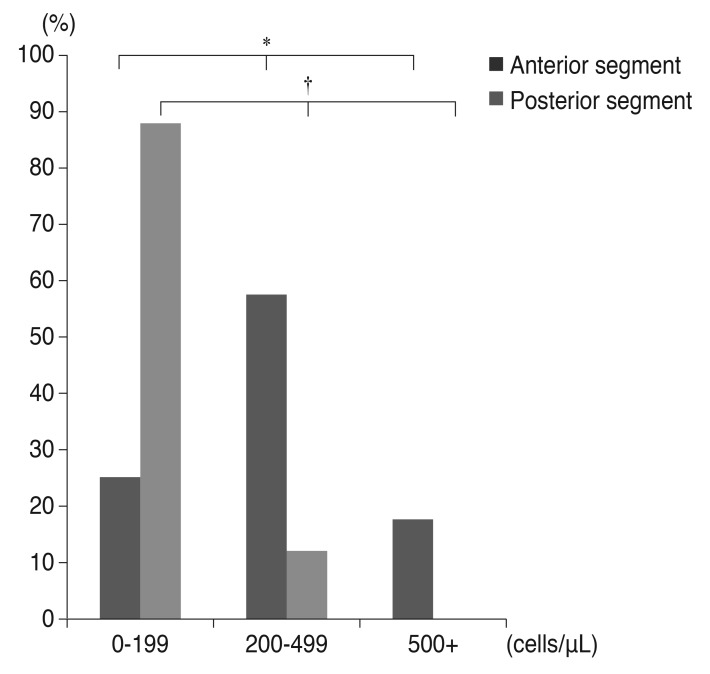
Relative frequencies (percentages) of ocular manifestations in patients grouped according to the CD4+ counts. *p < 0.05; †p < 0.01, chi-square test.
Anterior segment and adnexal ocular manifestations were seen in 36 patients (28.3%). The most common anterior segment ocular manifestation was keratoconjunctivitis sicca, which was seen in 14.2% of the patients, followed by conjunctival microvasculopathy (9.4%) and herpes zoster ophthalmicus (2.4%). The majority of patients with anterior segment ocular manifestations (58.3%) had CD4+ counts of 200 to 499 cells/µL. Keratoconjunctivitis sicca and conjunctival microvasculopathy were also common in patients with CD4+ counts of 200 to 499 cells/µL (Table 2).
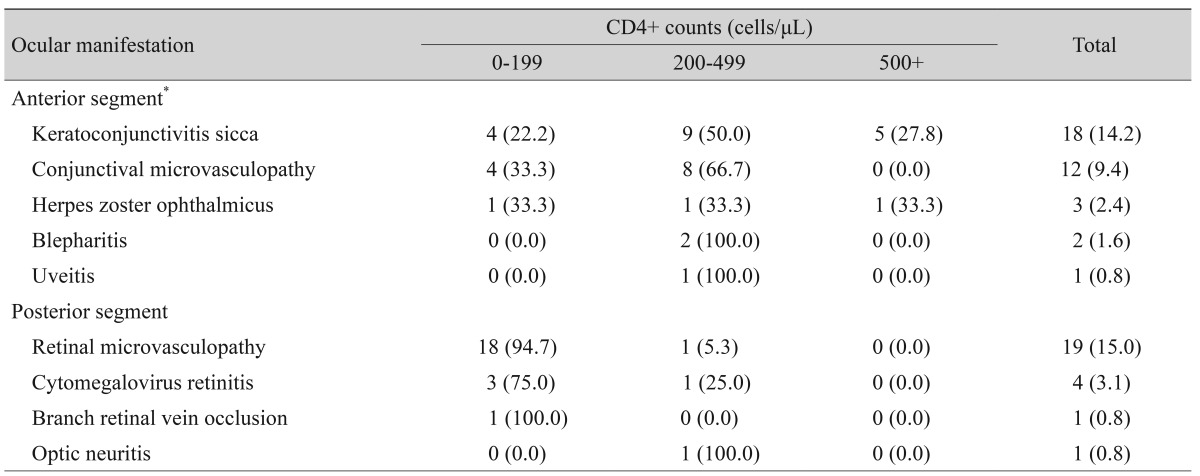
Distribution of the ocular manifestations and CD4+ counts among human immunodeficiency virus-infected or acquired immunodeficiency syndrome patients
Posterior segment ocular manifestations were seen in 25 patients (19.7%). Retinal microvasculopathy was seen in 19 patients (15.0%), followed by CMV retinitis (3.1%), branch retinal vein occlusion (0.8%), and optic neuritis (0.8%). One patient had retinal detachment present at the time of diag-nosis of CMV retinitis. Retinal microvasculopathy and CMV retinitis were common in patients with CD4+ counts <200 cells/µL. Other ocular manifestations, neoplasms, or opportunistic infections such as progressive outer retinal necrosis were not found in this study.
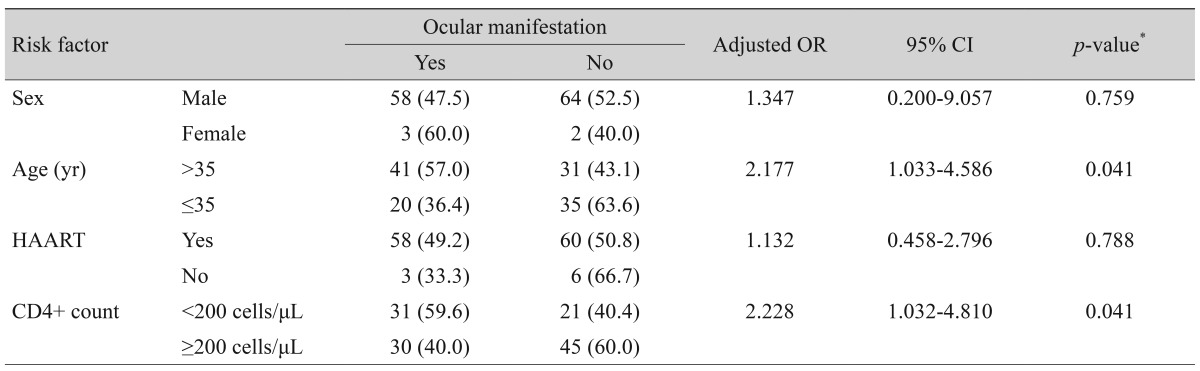
Multiple logistic regression was performed using variables that were significantly associated with the presence of ocular manifestations. Patients with CD4+ counts <200 cells/µL were more likely to have ocular manifestations than patients with CD4+ counts ≥200 cells/µL (p = 0.041; adjusted odds ratio [OR], 2.228; 95% confidence interval [CI], 1.032 to 4.810). Patients >35 years old were more likely to have ocular manifestations than patients age years (p = 0.041; adjusted OR, 2.177; 95% CI, 1.033 to 4.586). Notably, there were no significant associations between ocular manifestations and sex or HAART (p > 0.05) (Table 3).
Discussion
The overall incidence of ocular manifestations in this study was higher than in other studies performed in Asia, which had ranged from 15.8% to 45.0% [8212223242526272829]. This difference is likely due to the spectrum of ocular manifestations considered, as most articles did not include anterior segment manifestations such as keratoconjunctivitis sicca and conjunctival microvasculopathy. As the general health of individuals with HIV infection improves, the evaluation and management of disorders such as keratoconjunctivitis sicca and blepharitis, which were previously overshadowed by more severe, blinding disorders, may demand increased attention.
Keratoconjunctivitis sicca appears to be more common among individuals with AIDS (16.9% to 38.8%) [303132]. Prevailing theories of its pathogenesis implicate HIV itself as the inflammatory mediator that destroys primary and accessory lacrimal glands. Direct infection and damage to the conjunctiva may also be involved [15]. Blepharitis is also common in HIV-infected individuals, and blepharitis and an eyelid ulcers have been reported to be the initial manifestations of HIV disease in some patients [33]. The pathogenesis of blepharitis in immunodeficient individuals may either involve a reduced ability to control normal flora or more complex changes in cutaneous glands of the eyelids that occur with immunosuppression [34].
The use of HAART seeks to inhibit the progression to AIDS and to prevent death by reducing plasma HIV RNA to sustained low levels, thereby bolstering immunity [35]. An enhanced immune function has been documented in the form of decreased HIV-associated opportunistic infections with associated reductions in ocular morbidity. Contrary to its effect on many other ocular HIV/AIDS-related diseases, HAART has not played a part in significantly reducing the prevalence of keratoconjunctivitis sicca. According to a recent study, 17.8% of HIV-infected individuals given HAART still presented with keratoconjunctivitis sicca after treatment [35].
With HAART, patients are less likely to be affected by blinding posterior segment infections. However, attention should be paid to minor problems requiring ophthalmic care, including anterior segment and external ocular manifestations, which can affect the quality of life [2]. In this study, the incidence of anterior segment manifestations was higher than that of posterior segment manifestations. The most common anterior segment ocular manifestation was keratoconjunctivitis sicca, followed by conjunctival microvasculopathy, comprising a significant portion of the overall incidence. The incidence of keratoconjunctivitis sicca was similar to that in other studies, 21% in the pre-HAART era [31] and 17.8% in the post-HAART era [35]. However, the incidence of conjunctival microvasculopathy was much lower than in studies performed in the pre-HAART era (75%) [9363738]. Contrary to its effect on many other ocular HIV/AIDS-related diseases, HAART has not significantly reduced the incidence of keratoconjunctivitis sicca [39].
The most common ocular manifestation in this study was retinal microvasculopathy. The incidence of retinal microvasculopathy was similar to that in previous studies (8% to 12.6%) [8212223242526272829]. However, the incidence of CMV retinitis was lower than the 5.3% to 20.8% reported in other studies [8212223242526272829]. Before the HAART era, CMV retinitis was diagnosed in 10% to 45% of patients with late-stage AIDS, and some autopsy series documented CMV in up to 90% of patients [40414243]. By contrast, in the HAART era, there has been an estimated 80% decrease in the incidence of CMV retinitis [44]. The use of HAART has decreased plasma levels of HIV RNA a nd increased CD4+ counts, improving immune function of patients with HIV infection [454647]. Immune recovery uveitis was first described in 1998 as a consequence of rapid immune reconstitution after the use of HAART in HIV patients [48]. It is characterized by the development of intraocular inflammation, cystoid macular edema, epiretinal membrane, and vitritis. However, there were no cases of immune recovery uveitis in our series.
In this study, the mean CD4+ count was significantly lower in patients with posterior segment ocular manifestations compared to anterior segment ocular manifestations. Posterior segment ocular manifestations were common in patients with CD4+ counts <200 cells/µL. It is known that the prevalence of retinal microvasculopathy is inversely proportional to the CD4+ count, and CMV retinitis is common in patients whose CD4+ counts are <50 cells/µL [49]. However, anterior segment manifestations were common in patients with CD4+ counts of 200 to 499 cells/µL. Keratoconjunctivitis sicca is not related to the CD4+ count or associated with the severity of HIV [50]. Conjunctival microvasculopathy was also common in patients with CD4+ counts <100 cells/µL [51]. Conversely, no correlation between CD4+ count and conjunctival microvasculopathy has been reported [52]. In this study, both keratoconjunctivitis sicca and conjunctival microvasculopathy were common in patients with CD4+ counts of 200 to 499 cells/µL.
In this study, CD4+ counts <200 cells/µL and age >35 years were independent risk factors for developing ocular manifestations, with CD4+ counts <200 cells/µL being a well-established risk factor [53]. Age >35 years was found to be an independent risk factor for developing ocular manifestations [54]. One idea behind this difference between age groups is that age may actually affect patient immunity.
One limitation of the present study is that we diagnosed keratoconjunctivitis sicca using only a subjective index, the OSDI questionnaire. Recently, ocular surface staining scores by the Oxford system [55], tear film breakup time, and the Schirmer-1 test scores were used as objective signs for diagnosing keratoconjunctivitis sicca. Another limitation of our study is its small population of AIDS patients not on HAART.
In conclusion, this study demonstrated that the introduction of HAART has changed the landscape of ocular presentations. The incidence of anterior segment and external ocular manifestations was higher than posterior segment manifestations, and the most common ocular manifestation in this study was retinal microvasculopathy, followed by keratoconjunctivitis sicca and conjunctival mirovasculopathy. Posterior segment manifestations were common in patients with CD4+ counts <200 cells/µL, while anterior segment manifestations were common in patients with CD4+ counts of 200 to 499 cells/µL. Age >35 years was also found to be an independent risk factor for developing ocular manifestations.
Acknowledgements
This work was supported by the Soonchunhyang University Research Fund.
Notes
Conflict of Interest: No potential conflicts of interest relevant to this article was reported.