 |
 |
| Korean J Ophthalmol > Volume 28(3); 2014 > Article |
Abstract
Purpose
To evaluate the clinical efficacy of newly developed guidelines for the diagnosis and management of dry eye.
Methods
This retrospective, multi-center, non-randomized, observational study included a total of 1,612 patients with dry eye disease who initially visited the clinics from March 2010 to August 2010. Korean guidelines for the diagnosis and management of dry eye were newly developed from concise, expert-consensus recommendations. Severity levels at initial and final visits were determined using the guidelines in patients with 90 ┬▒ 7 days of follow-up visits (n = 526). Groups with different clinical outcomes were compared with respect to clinical parameters, treatment modalities, and guideline compliance. Main outcome measures were ocular and visual symptoms, ocular surface disease index, global assessment by patient and physician, tear film break-up time, Schirmer-1 test score, ocular surface staining score at initial and final visits, clinical outcome after three months of treatment, and guideline compliance.
Results
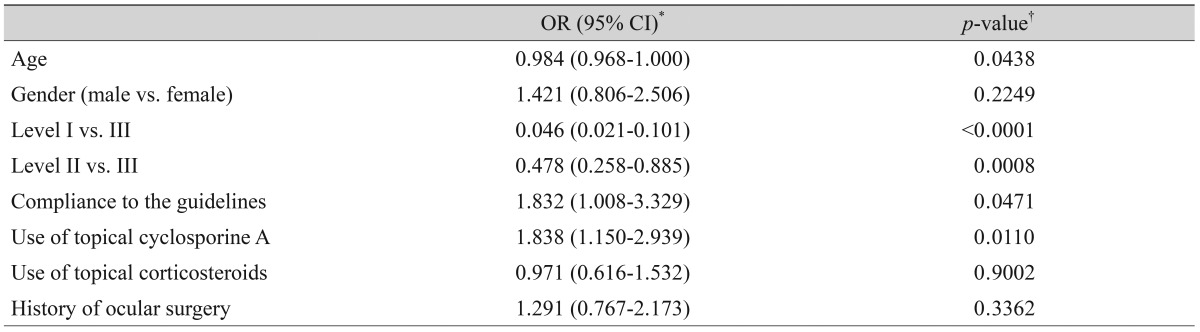
Severity level was reduced in 47.37% of patients treated as recommended by the guidelines. Younger age (odd ratio [OR], 0.984; p = 0.044), higher severity level at initial visit, compliance to treatment recommendation (OR, 1.832; p = 0.047), and use of topical cyclosporine (OR, 1.838; p = 0.011) were significantly associated with improved clinical outcomes.
The definition of dry eye disease has evolved due to a better understanding of the pathogenic mechanisms from recent research findings [1,2,3]. Contemporary concepts of dry eye disease describing the role of ocular surface inflammation as a major triggering factor and also as a therapeutic target is widely accepted since reports from the dysfunctional tear syndrome (DTS) study group and the International Dry Eye Workshop (DEWS) [2,3].
However, diagnosis of dry eye disease is sometimes challenging in practice, even with comprehensive diagnostic guidelines. Most diagnostic tests for dry eye disease lack reproducibility, and many emerging technologies such as the tear osmolarity test, interferometry, and meibometry or meibography are not widely used [4]. There is often a large discrepancy between a patient's symptoms and observed signs [5,6,7,8], and dry eye symptoms overlap with those of many other ocular surface diseases.
Previously, we surveyed patterns of dry eye disease observed in clinical practice by domestic ophthalmic practitioners who subspecialize in dry eye disease (unpublished data). We found that most of these practitioners regarded patients' symptoms and signs of ocular surface inflammation as the two most important factors in the diagnosis and treatment of dry eye in patients. Therefore, we aimed to propose simple, new dry eye guidelines that focus on the two aforementioned factors.
The aim of this study was to develop concise and intuitive guidelines for the diagnosis and management of dry eye disease for general and specialized ophthalmologists, and to evaluate the clinical efficacy of these guidelines.
The Korean Corneal Disease Study Group (KCDSG) is an independent, non-profit, academic society whose members comprise the most active corneal subspecialists in Korea. In 2009, the initial survey on the definition, diagnosis, severity grading, and management of dry eye disease was conducted among the members of the KCDSG. In this survey, we found that 78.8% of KCDSG members use the DEWS classification [3] as diagnostic guidelines of dry eye disease, while 21.2% use guidelines proposed by the DTS group [2]. KCDSG members also responded that they consider subjective symptoms, tear film breakup time (TBUT), and signs of ocular surface inflammation of more diagnostic value than other parameters. Based on the results of this survey, along with a review of the contemporary literature with regard to definition, classification, and treatment recommendations for dry eye, the subcommittees held face-to-face meetings to reach a consensus on the issues related to the definition, diagnosis, severity grading, and treatment recommendations for dry eye disease. New guidelines for the diagnosis and management of dry eye disease were adopted as shown in Tables 1 and 2. These guidelines were based on DEWS guidelines and modified to simplify the grading scheme so that they could be used more easily in clinical practice.
Dry eye disease was defined as "a disease of the ocular surface that is associated with tear film abnormalities." We agreed that a patient should be diagnosed with dry eye disease when he or she has at least one symptom and one objective sign. In the diagnosis guidelines, dry eye symptoms included ocular symptoms (such as dryness, discomfort, foreign body sensation, and pain) and visual symptoms (such as blurring or vision fluctuation). Ocular surface staining score by the Oxford system [9], TBUT, and Schirmer-1 test score were used as objective signs for diagnosing dry eye disease. Conjunctival injection, lid problems such as blepharitis, trichiasis, keratinization, or symblepharon, and tear film abnormalities such as debris, decreased tear meniscus, and mucus clumping, were considered signs of ocular surface inflammation, but these findings were not considered during the grading of disease severity. The severity level of the disease was determined when both designated symptoms and signs were present at a certain level. If there was a discrepancy between the patients' symptoms and signs, the severity level was determined according to the severity level of the objective signs. When several objective signs were present at different levels, the severity level of the disease was determined following ocular surface staining. In addition, we introduced a provisional category of "dry eye suspect," which is not listed on the grading scheme. The patient was diagnosed with suspected dry eye when he or she had only dry eye symptoms without any objective signs. This was to evaluate the distribution of disease severity at the initial visit, and the treatment recommendation did not include the category of "dry eye suspect."
Detailed treatment options for each particular level from level I to level IV were recommended as shown in Table 2.
We conducted a multicenter, retrospective observational study to validate the clinical efficacy of the developed guidelines for the diagnosis and management of dry eye. The main interests were: 1) distribution of disease severity levels according to the guidelines, 2) correlation between severity level and clinical parameters, 3) current practice pattern and compliance to the management recommendations, and 4) factors that affect clinical outcomes.
Institutional review board approval was obtained, and the study complied with the tenets of the Declaration of Helsinki. The medical records of dry eye patients who initially visited any of the 37 of 50 KCDSG member institutes (listed in the Acknowledgements) from March to August 2010 were reviewed. Patients were included regardless of any previous treatment history of dry eye disease. Subjective and objective assessments, dry eye therapies, and guideline compliance were analyzed. Subjective assessments included ocular symptoms, visual symptoms, ocular surface disease index (OSDI) [10], and global assessment by the patient on a scale of 0 to 5, with 0 being the best and 5 the worst. Objective assessments included the Schirmer-1 test score, TBUT, ocular surface staining score by the Oxford scale, and global assessment by the physician on a scale of 0 to 5, with 0 being the best and 5 the worst. Severity level of dry eye disease was determined by the grading scheme of the proposed guidelines.
Among the patients enrolled, those who had a follow-up visit at 90 ┬▒ 7 days were analyzed to validate the clinical efficacy of the developed guidelines. For the purpose of analysis, "improvement" was defined as a decrease in the severity level of dry eye according to the proposed grading scheme at the final visit. "Compliant" was defined as selection of initial treatment modality according to the developed guidelines. "Under-treatment" was defined as 1) selection of an initial treatment modality from among the treatments available that is normally recommended for a milder level of disease and 2) use of improper level of treatment modality, with no improvement in disease level. "Over-treatment" was defined as selection of an initial treatment modality from among the treatments recommended that was more appropriate for a higher (more severe) level of disease.
The main outcome measure was the clinical outcome of dry eye after three months of treatment and factors affecting outcome, including compliance to the KCDSG guidelines.
All statistical analyses were performed using SAS ver. 9.1 (SAS Inc., Cary, NC, USA). A t-test was used to analyze the differences in subjective and objective parameters before and after treatment. Maximum likelihood estimation of logistic regression models was used to analyze factors contributing to the clinical outcome. A chi-squared test and Fisher's exact test were used to analyze the association of compliance with the guidelines and clinical outcome.
The medical records of 1,691 patients were reviewed. Excluding 79 cases whose clinical information was insufficient, data on 1,612 patients were analyzed during the initial visit. The mean age was 56.9 (┬▒13.2) years (range, 19 to 85 years), and 81% were female. Distribution of severity level at the initial visit is summarized in Fig. 1. Since only eight patients were considered level 4 by the diagnosis guidelines, the severity of level 3 and level 4 were grouped together (n = 68) for the statistical analysis.
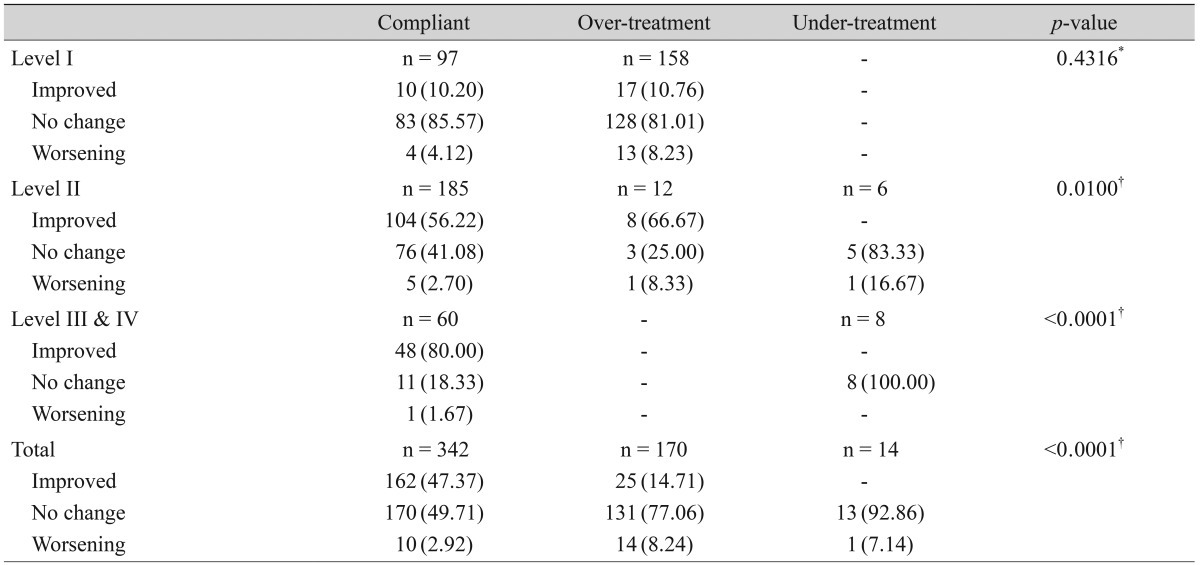
Of the 1,691 patients, 526 met the follow-up visit criteria and were included for analysis of clinical outcome. The demographic and clinical characteristics of these patients are summarized in Table 3. Treatment was carried out according to the guideline recommendations in 65.0% (342 / 526) of these patients. Over-treatment was evident in 32.3% (170 / 526) of patients, while under-treatment was evident in 2.7% (14 / 526).
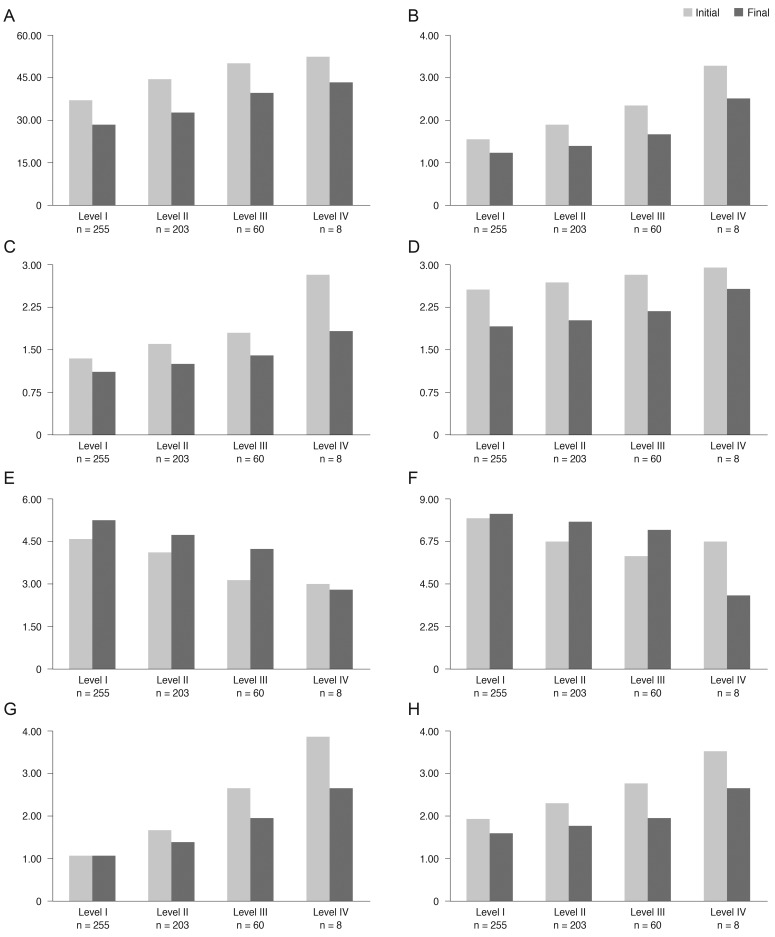
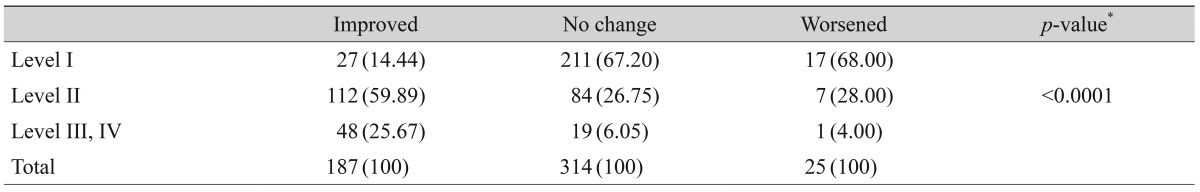
After three months of treatment, the severity level decreased in 35.6% (187 / 526) of patients, did not change in 59.7% (314 / 526) of patients, and increased in 4.8% (25 / 526) of patients. Fig. 2 shows the distribution of severity level at the initial and final visits. At three months, the proportion of level I dry eye increased from 48% to 63% of total patients, and objective signs of dry eye completely resolved with subjective symptoms persisting in 7% of patients (Fig. 2). Subjective and objective parameters also improved from baseline in this cohort (Fig. 3). Logistic regression analysis showed that younger age, higher severity level at the initial visit, compliance with the treatment recommendations, and use of topical cyclosporine were significantly associated with improvement in clinical outcomes (Table 4). We also found that distributions of severity level at the initial visit were significantly different among clinical outcome groups (chi-square test, p < 0.0001) (Table 5).
Discordant with treatment recommendations, therapeutic modalities for severity level II or above were used for 62.0% (158 / 255) of level I dry eye patients. However, clinical outcome was not signif icantly improved in the over-treatment group compared to the compliant group in level I patients (10.20% vs. 10.76% improvement, p = 0.4316). Overall, 47.37% of the patients in the compliant group showed improvement, while 14.71% in the over-treatment group showed improvement (p < 0.0001) (Table 6).
The Korean guidelines for the diagnosis and management of dry eye are concise, practice-focused, expert consensus recommendations that are simple, intuitive, and easy-to-use in a clinical practice setting. In developing these guidelines, contemporary concepts of diagnosis, grading, and management of dry eye disease were adopted and modified [1,2,3].
The members of the KCDSG agreed that dry eye disease should be diagnosed when a patient has at least 1 sign and symptom. However, symptoms may precede signs in certain patients, and it is difficult to exclude short-term fluctuations in symptoms and signs. Therefore, we categorized the presence of only dry eye symptoms without ocular signs as suspected dry eye. A patient with suspected dry eye was not listed in the grading system because the general consensus was that dry eye treatment should not be recommended for these patients.
The grading system is adopted and modified from the dry eye severity grading scheme of the DEWS [3]. We gave much weight to patients' symptoms and ocular surface staining scores in grading the disease severity. Ocular signs, such as conjunctival injection, lid abnormalities, and tear film abnormalities were not considered in the grading system because these are common findings in other ocular surface diseases and often overlap with dry eye disease [11]. However, these findings may be regarded as positive ocular signs in the diagnosis of dry eye.
The Schirmer-1 test score represents the patient's reflex tear flow and it is used to diagnose aqueous-deficient dry eye disease [4]. However, there is wide intra-patient variation in the test results, and the test is not routinely performed on every dry eye patient in the office. The interesting finding in this study was that no case was diagnosed only by a low Schirmer-1 test score without any other objective signs. The patients with low Schirmer scores were always accompanied by positive findings in other objective parameters. This result implies that the Schirmer-1 test may not be a mandatory parameter in the diagnosis of dry eye disease, although it is useful in the evaluation of tear function.
A stepladder approach according to severity level was recommended to treat dry eye disease. We recommended non-preserved artificial tears for dry eye patients with level II severity or greater and anti-inflammatory therapy for those with level II severity or greater.
This retrospective observational study included 1,612 patients at the initial visit. More than 50% of the patients had level I severity or less (47.5% patients had level I severity, and 8.8% had suspected dry eye), and 33.5% had level II severity. Only 32.6% (526 / 1,612) of the total patients were eligible to be included in the analysis of clinical outcomes. High drop-out rates during the follow-up period may raise concerns about selection bias for severe cases. However, distribution of the severity level of these 526 patients was not significantly different from that of the initial visit group. Cases of suspected dry eye at the initial visit were excluded from the clinical analysis because the guidelines did not recommend any treatment for such patients.
This study showed that the severity level according to our guidelines correlated well with other subjective and objective parameters, including OSDI, patient's global assessment, and physician's global assessment. We also found that treatment based on these guidelines results in better clinical outcomes. Although the participating physicians were aware of the treatment recommendation guidelines, they were free to choose treatment modalities using the guidelines and their own clinical judgment. An interesting finding was that many patients with level I severity were treated with therapeutic modalities recommended for higher severity levels, which included mostly anti-inflammatory therapy, and this failed to result in better outcomes. Wide use of anti-inflammatory therapy for level I severity patients may be attributed to the fact that most of the participating institutions were referral hospitals. Wilson and Stulting [12] also reported that many practitioners treated DTS severity level 1 with therapies for DTS level 2. Although symptomatic improvement has been reported with anti-inflammatory treatments for mild dry eye [13], most of the treatment guidelines [2,14] list topical steroids or topical cyclosporine A as treatments for level II or greater [15,16,17].
Among anti-inflammatory treatments, clinical improvement was significantly higher with the use of topical cyclosporine A, but not with topical steroids. Since we analyzed the data at the initial and final visits only, the effect of topical steroids could have been masked because it is not likely that topical steroids were used during the entire follow-up period.
For the purpose of statistical analysis, we defined "improvement" as a decrease in severity level at the final visit. This definition evidently limited the clinical outcome of level I patients, because these patients were required to fall into the category of suspected dry eye at the final visit to show improvement. However, when the treatment guidelines were recommended based on disease severity level, it was not possible to assign clinical significance to any changes in subjective and objective parameters within the same severity level.
Evaluation of individual treatment modalities contributing to clinical outcomes was limited by the retrospective, non-randomized nature of the study. These contributions should be further evaluated by prospective and randomized research.
In conclusion, the Korean guidelines for the diagnosis and management of dry eye can be used as a valid and effective tool for the treatment of dry eye disease in clinical practice.
Acknowledgements
This study was supported by an unrestricted educational grant from Allergan (Irvine, CA, USA). The sponsor or funding organization had no role in the design or conduct of this research.
Listed members of the Korean Corneal Disease Study Group who were not listed as authors, made valuable contributions to this study (listed in alphabetical order by last name): Beom Jin Cho (HanGil Eye Hospital), Kee Yong Choi (HanGil Eye Hospital), Si Hwan Choi (Chungnam National University Hospital, Chungnam National University School of Medicine), Tae Hoon Choi (Nune Eye Hospital), Yeoun Sook Chun (Chung-Ang University Hospital, Chung-Ang University College of Medicine), So-Hyang Chung (Seoul St. Mary's Hospital, The Catholic University of Korea School of Medicine), Sung Kun Chung (Yeouido St. Mary's Hospital, The Catholic University of Korea School of Medicine), Tae Young Chung (Samsung Medical Center, Sungkyunkwan University School of Medicine), Tae Won Hahn (Apgujeong St. Mary's Eye Center), Young Ho Hahn (Han's Eye Center), Young Keun Han (Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul National University College of Medicine), Kyung Hyun Jin (KyungHee University Medical Center, KyungHee University School of Medicine), Choun-Ki Joo (Seoul St. Mary's Hospital, The Catholic University of Korea School of Medicine), Roo Min Jun (Ewha Womans University Mokdong Hospital, Ewha Womans University School of Medicine), Eun Chul Kim (Bucheon St. Mary's Hospital, The Catholic University of Korea School of Medicine), Eung Kweon Kim (Severance Hospital, Yonsei University College of Medicine), Gi Bong Kim (Plus St. Mary's Eye Center), Hong Kyun Kim (Kyungpook National University Hospital, Kyungpook National University School of Medicine), Hyun Seung Kim (Yeouido St. Mary's Hospital, The Catholic University of Korea School of Medicine), Hyung Joon Kim (Daegu Catholic University Medical Center, Catholic University of Daegu College of Medicine), Jae Chan Kim (Chung-Ang University Hospital, Chung-Ang University College of Medicine), Jin Hyoung Kim (Inje University Ilsan Paik Hospital, Inje University College of Medicine), Ki San Kim (Korea Kim Ki San Eye Center), Man Soo Kim (Seoul St. Mary's Hospital, The Catholic University of Korea School of Medicine), Mee Kum Kim (Seoul National University Hospital, Seoul National University College of Medicine), Su Young Kim (Uijeongbu St. Mary's Hospital, The Catholic University of Korea School of Medicine), Tae-im Kim (Severance Hospital, Yonsei University College of Medicine), Woo Jung Kim (Seoul Samsung Eye Clinic), Jae Woong Koh (Chosun University Hospital, Chosun University School of Medicine), Ji Won Kwon (Myongji Hospital, Kwandong University College of Medicine), Ji Eun Lee (Pusan National University Yangsan Hospital, Pusan National University College of Medicine), Ha Bum Lee (Kang Dong Sacred Heart Hospital, Hallym University College of Medicine), Hyung Keun Lee (Gangnam Severance Hospital, Yonsei University College of Medicine), Jin Hak Lee (Seoul National University Bundang Hospital, Seoul National University College of Medicine), Jong Hyuck Lee (Wonju Christian Hospital, Yonsei University Wonju College of Medicine), Jong Soo Lee (Pusan National University Hospital, Pusan National University School of Medicine), Sang-Bumm Lee (Yeungnam University Medical Center, Yeungnam University College of Medicine), Woo Chan Park (Dong-A University Hospital, Dong-A University College of Medicine), Woo Jin Sah (Apgujeong St. Mary's Eye Center), Kyoung Yul Seo (Severance Hospital, Yonsei University College of Medicine), Hung Won Tchah (Asan Medical Center, University of Ulsan College of Medicine), Won Ryang Wee (Seoul National University Hospital, Seoul National University College of Medicine), Kyung Chul Yoon (Chonnam National University Hospital, Chonnam National University Medical School), In Cheon You (Chonbuk National University Hospital, Chonbuk National University Medical School).
REFERENCES
1. Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. CLAO J 1995;21:221-232.

2. Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea 2006;25:900-907.


3. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5:75-92.


4. Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5:108-152.


5. Han SB, Hyon JY, Woo SJ, et al. Prevalence of dry eye disease in an elderly Korean population. Arch Ophthalmol 2011;129:633-638.


6. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye Work-Shop (2007). Ocul Surf 2007;5:93-107.


7. Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea 2004;23:762-770.


8. Nichols KK, Smith JA. Association of clinical diagnostic tests and dry eye surveys: the NEI-VFQ-25 and the OSDI. Adv Exp Med Biol 2002;506(Pt B):1177-1181.


9. Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003;22:640-650.


10. Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol 2000;118:615-621.


11. Nelson JD, Shimazaki J, Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci 2011;52:1930-1937.



12. Wilson SE, Stulting RD. Agreement of physician treatment practices with the international task force guidelines for diagnosis and treatment of dry eye disease. Cornea 2007;26:284-289.


13. Perry HD, Solomon R, Donnenfeld ED, et al. Evaluation of topical cyclosporine for the treatment of dry eye disease. Arch Ophthalmol 2008;126:1046-1050.


14. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5:163-178.


15. Baiza-Duran L, Medrano-Palafox J, Hernandez-Quintela E, et al. A comparative clinical trial of the efficacy of two different aqueous solutions of cyclosporine for the treatment of moderate-to-severe dry eye syndrome. Br J Ophthalmol 2010;94:1312-1315.


Fig.┬Ā1
Distribution of severity level of dry eye (DE) disease at the initial visit (n = 1,612) (Korean Corneal Disease Study Group guidelines).
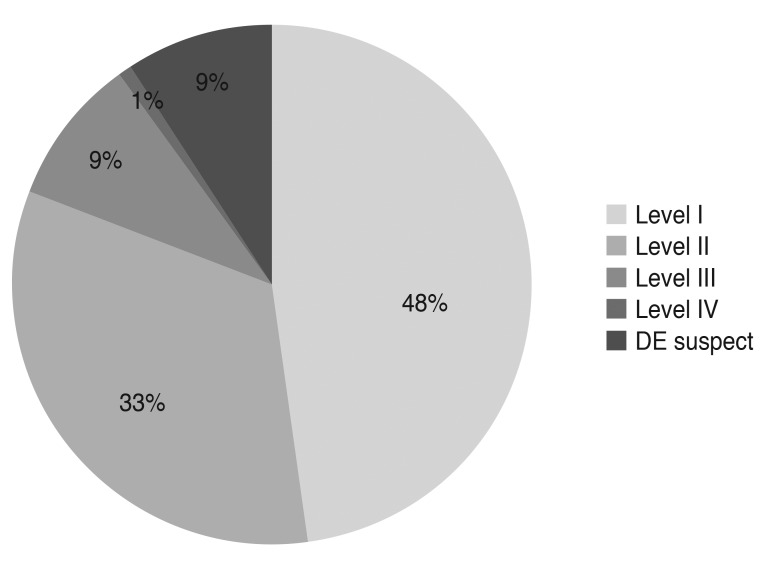
Fig.┬Ā2
Severity level of dry eye disease at the initial and final visits (n = 526) (Korean Corneal Disease Study Group guidelines).
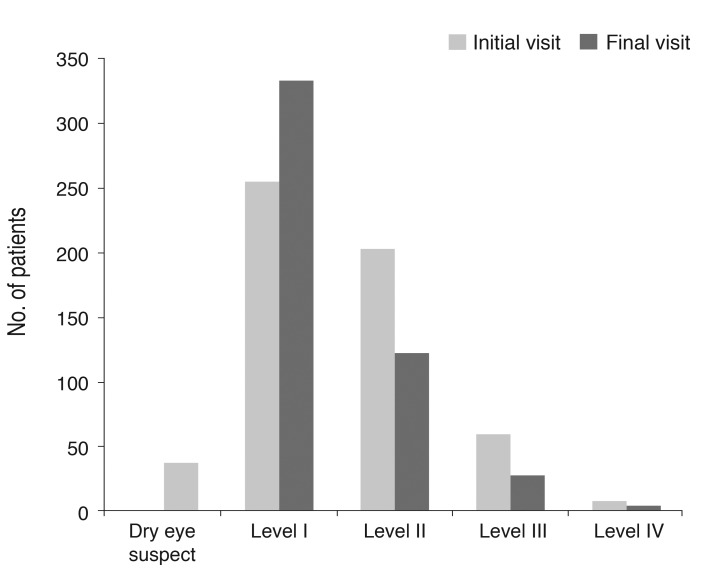
Fig.┬Ā3
Changes of subjective (A-D) and objective (E-H) clinical parameters in dry eye patients (n = 526) of each initial severity level (Korean Corneal Disease Study Group guidelines). (A) Ocular surface disease index (%), (B) irritation and visual symptoms (1, sometimes; 2, often; 3, always; 4, daily life limited), (C) visual symptom, (D) subjective global assessment (0 [best] to 5 [worst]), (E) tear film break up time (sec), (F) Schirmer-1 test score (mm/5 min), (G) ocular surface staining score (Oxford scale), (H) objective global assessment (0 [best] to 5 [worst]).
Table┬Ā1
Korean Corneal Disease Study Group guidelines for the diagnosis of dry eye disease

*Positive ocular signs may include conjunctival injection, lid abnormalities (blepharitis, trichiasis, keratinization, and symblepharon), and tear film abnormalities (debris, decreased tear meniscus, and mucus clumping). However, these findings are not considered in the grading of disease severity; ŌĆĀOxford system.





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




