Globally, the market for eye disease treatment and research and development has been growing, along with an increased incidence of various eye diseases due to a rapid increase in the aging population and worsening air pollution [1]. When eye drops are administered for the treatment of eye diseases, a low corneal penetration rate is achieved due to barriers and blinking of the eye. Moreover, the residence time of the drug on the corneal surface is only 10 minutes or less due to the discharge through the lacrimal duct, thereby leading to the inconvenience of applying the eye drops at a given frequency [2,3]. Thus, the treatment of eye diseases using eye drops has issues such as inconvenience and side effects due to high dosing frequency, drug waste, and low corneal permeability [4]. Therefore, it is necessary to develop a drug delivery system (DDS) that is capable of maintaining the intraocular drug concentration as well as improving the drug absorption.
Recently, materials that can replace organs and tissues using 3-dimentional (3D) bioprinting technology have been increasingly developed for this purpose. Bioprinting using bio-ink is a technology that creates a material that can function as a tissue or an organ of the human body [5-12]. In this study, a new lens-type biodegradable DDS was manufactured using 3D bioprinting to make treatment convenient, increase drug contact time, and heal the epithelium in patients with dry eye.
Materials and Methods
Ethics statement
This study was conducted according to the Declaration of Helsinki after approval from the institutional review board of Chosun University Hospital (2019-03-001). Informed consent was waived from institutional review board due to non-clinical study (in vitro).
Fabrication of DDS
A new lens-type biodegradable DDS was modeled using a Bio X 3D Bioprinter (Cellink, Boston, MA, USA). The temperature of the printer head and bed was controlled, and the pressure and speed of the nozzle were controlled during secreting. A new lens-type biodegradable DDS was manufactured using gelatin methacryloly as a base for the formation of framework and 0.1%, 0.15%, and 0.3% hyaluronic acid and tobramycin as bio-inks. The printer head temperature was set to 26┬░C and the bed temperature was set to 9┬░C. The pressure and speed were adjusted during discharge to measure the lens shape maintenance and ease of design. The basic modeling values for the lens were diameter, 15 mm; height, 4 mm; and thickness, 0.3 mm, and after fabrication, each size was measured to confirm whether it was consistent with the initial setting value.
Determination of antibiotic concentrations
To identify an effective concentration of an antibiotic that causes less apoptosis when in contact with cells, different concentrations of tobramycin were mixed with live cells (human conjunctival epithelial cells) to evaluate cell survival and apoptosis. MTT (3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl-2H-tetrazolium bromide) assay was performed to determine cell viability.
Measurement of degradation of DDS
The degree of degradation of the new lens-type biodegradable DDS manufactured through the above procedure was measured. The weight of the initially manufactured material was first measured using an electronic scale, and the weight remaining after degradation was subsequently measured over time. Three solutions of 0.1%, 0.15%, and 0.3% concentrations of hyaluronic acid were prepared to measure the degree of degradation according to varying concentrations of hyaluronic acid. Further, the material was stored in phosphate-buffered saline (PBS) at 4┬░C and 37┬░C, and the weight was measured to determine the degree of degradation according to temperature. The post-processing time of ultraviolet (UV) crosslink ranged from 0 to 120 seconds, and the degree of degradation was measured accordingly.
Results
The new lens-type biodegradable DDS was designed with the following initial settings of the 3D printer: printer head temperature, 26┬░C; printer bed temperature, 9┬░C; diameter, 15 mm; height, 4 mm; and thickness, 0.3 mm. The DDS was created with the same values as the initial modeling values, and the shape was maintained when using a nozzle pressure of 80 kPa and speed of 4 mm/sec for 0.1% hyaluronic acid, a nozzle pressure of 50 kPA and speed of 3 mm/sec for 0.15% hyaluronic acid, a nozzle pressure of 60 kPA and the speed of 8 mm/sec for 0.3% hyaluronic acid (Fig. 1A, 1B). In conditions other than the optimal ones, either the diameter of the lens increased to 17-20 mm or the height decreased to 2.5-3.0 mm, and the shape was not maintained after production.
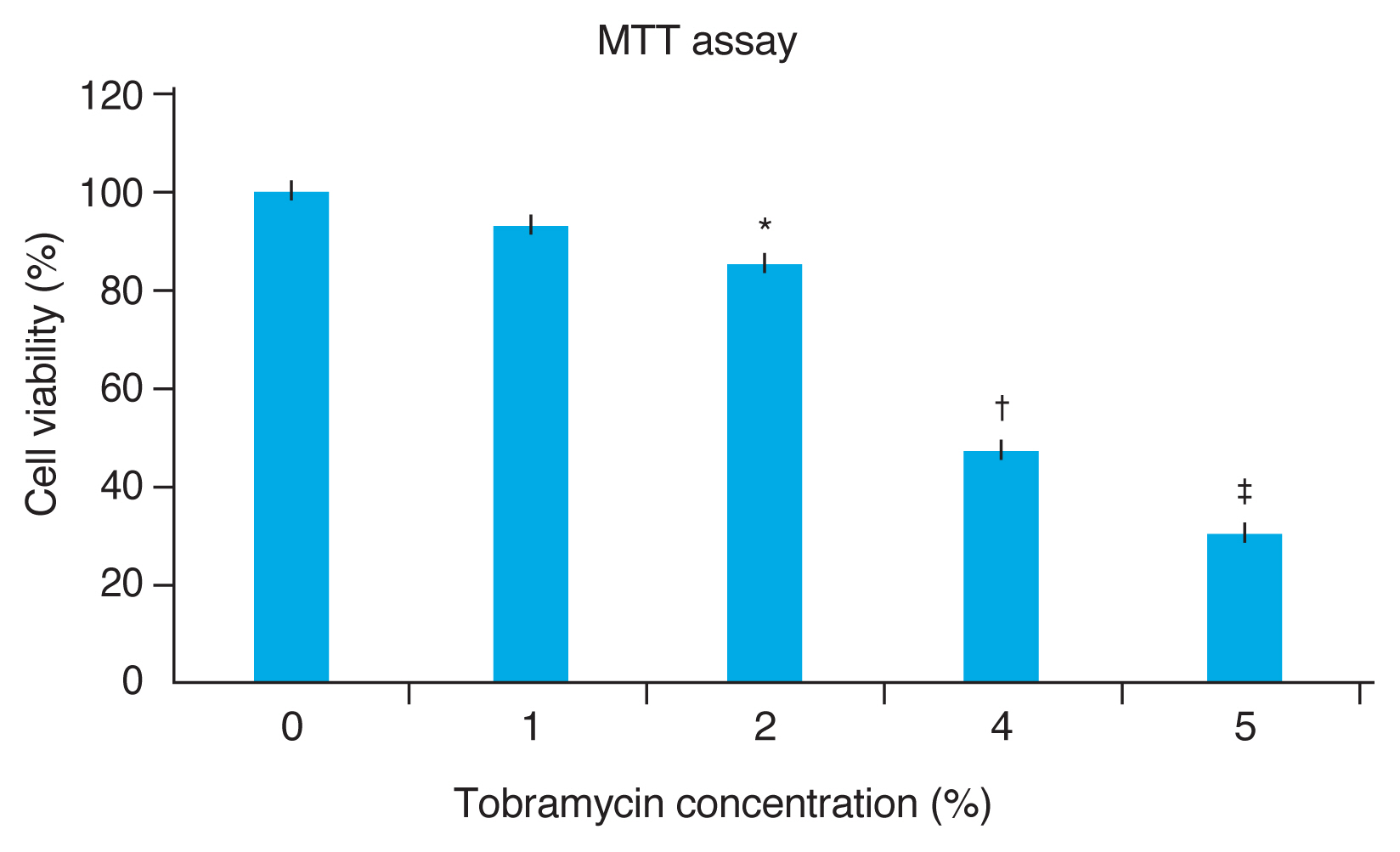
Cell viability according to the concentration of tobramycin was confirmed through MTT assay. When 1% tobramycin and live cells were mixed, more than 90% of the normal cells survived (p > 0.05). At 2%, 4%, and 5% tobramycin, the survival rate was 84% (p < 0.05), 52% (p < 0.005), and 31% (p < 0.005) respectively. In other words, cytotoxicity was shown at a concentration of 2% tobramycin (p < 0.05). Since no cytotoxicity was observed at 1% tobramycin (p > 0.05), and pharmacological effects were as clinically expected at this concentration, it was determined to be appropriate (Fig. 2).
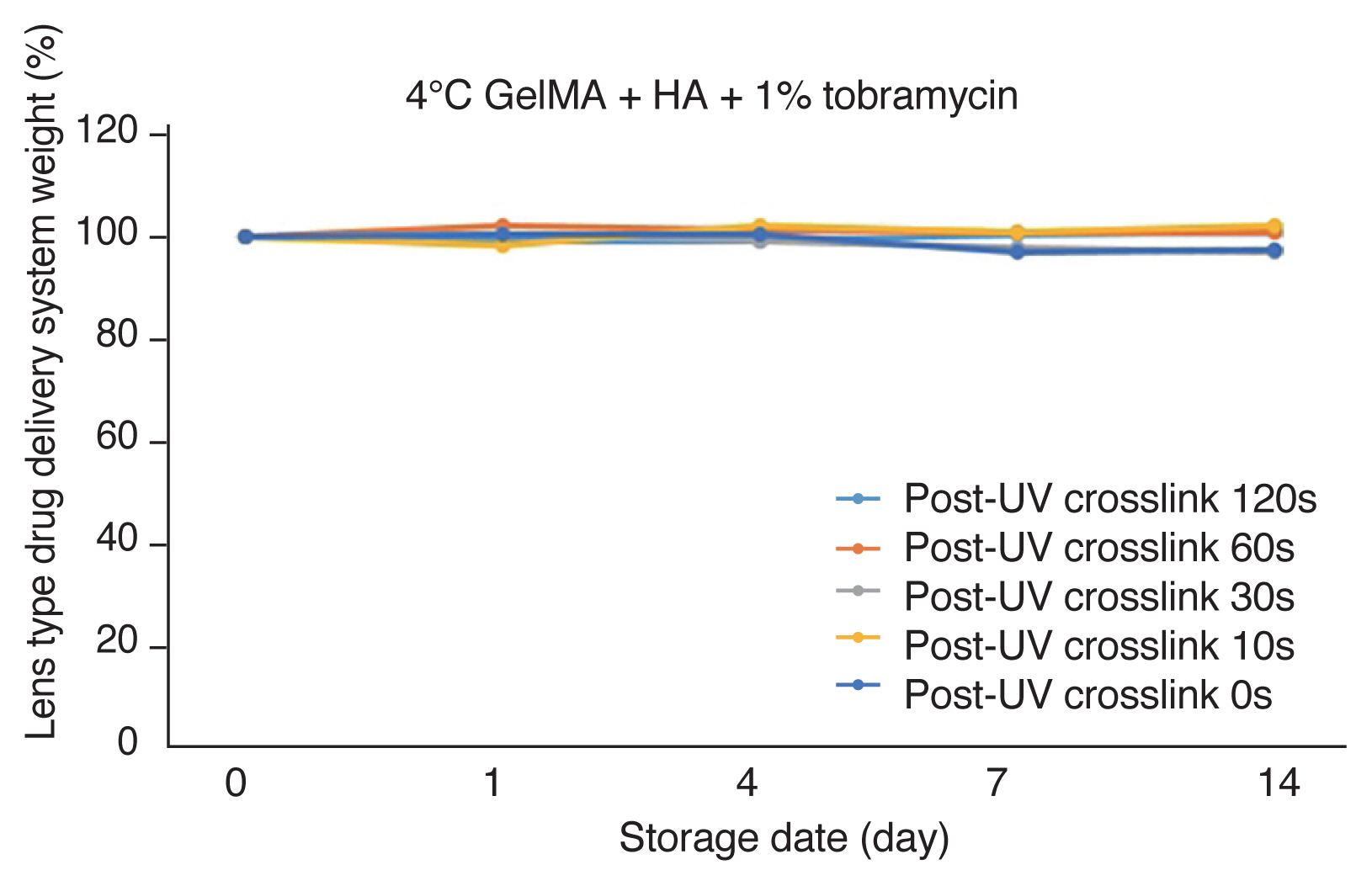
When the new lens-type biodegradable DDS manufactured under the most appropriate conditions above was stored in PBS at 4┬░C, it was confirmed that biodegradation did not occur regardless of the UV crosslink post-processing and the concentration of hyaluronic acid (Fig. 3). In other words, it was found that it could be stored continuously at 4┬░C.
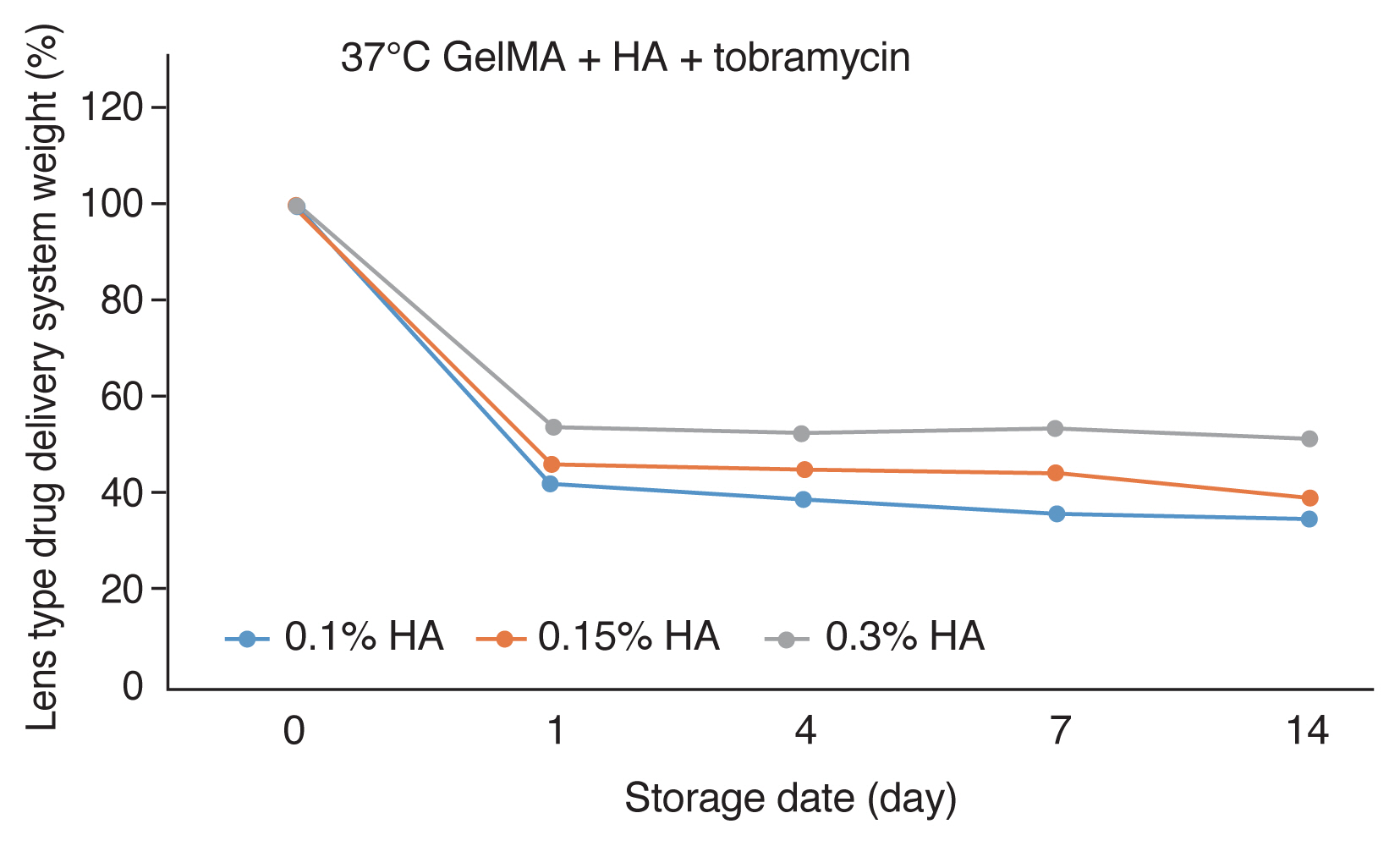
Biodegradation was shown when the new lens-type biodegradable DDS made of different concentrations of hyaluronic acid was stored in PBS at 37┬░C after 60 seconds of UV crosslink post-processing. Degradation of up to 42%-54% occurred within the first 24 hours, and the DDS remained at a similar concentration until 14 days thereafter. As a result, when the DDS was stored at 37┬░C and UV crosslink post-processing was performed for 60 seconds, it was stored at a constant level for more than 14 days regardless of the concentration of hyaluronic acid (Fig. 4).
Discussion
Bio-ink is a biomaterial that transports living cells during bioprinting. It refers to materials that can be applied to bioprinting technology to produce necessary structures, including living cells and biomolecules. Bio-ink material requires excellent biocompatibility to be used in bioprinting and physical properties that can be printed in the desired pattern by smoothly passing through a micro-diameter nozzle, and it should be able to maintain its role as a mechanical support while providing a cell-specific signal after printing [13-15]. Currently, bio-inks are being actively studied to produce substitute materials for organs and tissues. It is possible to manufacture bio-inks as a structure, but there is still a limitation with regard to their functional role. For example, heart replacement material can be manufactured so that it can function almost identically similar to the real one in terms of structure. However, there is a limit to its role in making and transmitting the electric current of cardiomyocytes in terms of its functional aspect [16,17]. Bio-inks currently widely use include gelatin, hyaluronic acid, collagen, fibrinogen, and glycerol. These materials are hydrophilic polymers cross-linked by cohesive force and have a 3D polymer network structure that can absorb a large amount of water and swell in an aqueous solution. In addition, these may be biodegradable and biocompatible materials, and degradation and transparency can be controlled according to concentration. In the field of ophthalmology, there is a report on the replacement of the cornea using bioprinting [18]. In this study, the new lens-type biodegradable DDS was manufactured using gelatin-based gelatin methacryloly and hyaluronic acid. Hyaluronic acid is a hydrophilic substance that is abundantly present in the eyes and joints. In addition, it is possible to control its degree of degradation by changing the structure according to temperature [19]. Using these features, it can be stored at 4┬░C normally, and it can be manufactured to an appropriate level even at 37┬░C, which is the body temperature. Moreover, in this study, 1% tobramycin was mixed to prevent infection, thereby showing the possibility that other eye drops, as well as antibiotics, can be mixed when manufacturing such lenses. Since a constant concentration was maintained during biodegradation, it is expected that it can be manufactured successfully to treat various types of eye diseases. It is a patient-customized DDS using 3D bioprinting technology. It is formed according to the curves to make it easier on the cornea when worn, and the surface of the structure is slightly irregular but soft. In particular, when worn on the human body and exposed to tears, it is reached to the decomposition temperature since drug secretion progresses. Also, transparency and surface smoothness are increased by tears due to the nature of bio-ink. Still, more research is needed to secure the visibility of the lens and maintain the drug at a specific concentration after degradation. However, a new lens-type biodegradable DDS can be mass-produced quickly and easily using a 3D printer. The material produced in this way has excellent adhesion to the eye, biocompatibility, and bio absorption and increased adhesion time, thereby confirming the possibility of treating various diseases as well as dry eye disease, which is a meaningful result of this study.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print






