 |
 |
| Korean J Ophthalmol > Volume 26(2); 2012 > Article |
Abstract
Purpose
To evaluate the efficacy and safety of a pars plana Ahmed valve implantation combined with 23-gauge sutureless vitrectomy in the treatment of patients with medically uncontrolled neovascular glaucoma (NVG) in proliferative diabetic retinopathy (PDR).
Methods
The authors retrospectively reviewed the records of 11 consecutive patients with refractory NVG in PDR who underwent a 23-gauge sutureless vitrectomy combined with pars plana placement of an Ahmed valve implant. Control of intraocular pressure (IOP), pre- and postoperative best-corrected visual acuity and the development of intra- and postoperative complications were evaluated during the follow-up.
Results
The mean follow-up was 12.2 months (range, 8 to 25 months). Mean preoperative IOP was 35.9 ± 6.3 mmHg and mean postoperative IOP at the last visit was 13.3 ± 3.2 mmHg. Control of IOP (8 to 18 mmHg) was achieved in all patients, but 91% (10 of 11 patients) needed antiglaucoma medication (mean number of medications, 1.2 ± 0.6). Postoperative visual acuity improved in 11 eyes, and the logarithmically to the minimum angle of resolution mean visual acuity in these eyes improved from 1.67 ± 0.61 to 0.96 ± 0.67. The complications that occurred were transient hypotony in one case, transitory hypertension in two cases, and postoperative vitreous hemorrhage which spontaneously cleared in two cases.
Proliferative diabetic retinopathy (PDR) can cause severe visual loss due to the development of neovascular glaucoma (NVG) [1]. NVG in PDR often appears as a refractory condition that is not well controlled by any medical treatment. Glaucoma drainage implants have been used in the treatment of refractory glaucoma in PDR patients. Typically, the tube is placed in the anterior chamber and acts to shunt aqueous fluid to an equatorial implant [2,3]. NVG patients with PDR often subsequently experience vitreous hemorrhage or vitreous opacity, which interferes with conventional panretinal photocoagulation and may require vitrectomy. Moreover, the anterior chamber may not be the proper site for tube implantation in cases such as advanced glaucoma with secondary angle closure or angle neovascularization, corneal diseases, and other anterior chamber abnormalities. In such cases, the implant can be placed through pars plana combined with vitrectomy [4-7].
Several studies have showed pars plana vitrectomy with implantation of a drainage tube into the vitreous cavity achieved a success rate comparable to the success rate of an anterior chamber implant in refractory NVG [8-12]. Recently, 23 or 25-gauge sutureless vitrectomy has become more popular than 20-gauge vitrectomy. The small gauge vitrectomy has potential advantages over traditional 20-gauge pars plana vitrectomy, which includes improved healing time, increased postoperative comfort and diminished disturbance of the conjunctiva particularly with regard to trabeculectomy sites [13].
The purpose of this retrospective case series was to report on the outcome of pars plana Ahmed valve implantation combined with 23-gauge pars plana vitrectomy in patients with uncontrolled NVG in PDR.
The study was approved by the institutional review board of Gil Medical Center and adhered to the Declaration of Helsinki. The charts of PDR patients who had uncontrolled neovascular glaucoma which was unresponsive to medical treatment and underwent 23-gauge sutureless vitrectomy combined with Ahmed valve implantation by the same vitereoretinal surgeon (DYL) between March 1, 2008, and January 31, 2010, were reviewed retrospectively. Eleven patients met the criteria of this study (NVG with an intraocular pressure [IOP] ≥30 mmHg despite maximum tolerated oral and topical antiglaucoma medical therapy, dense vitreous opacity or vitreous hemorrhage and underlying retinal pathology [diabetic retinopathy]). Data collected included demographic information, indications for combined procedure, complications, preoperative and postoperative best-corrected visual acuity, and IOP (which was measured with a Goldmann applanation tonometer).
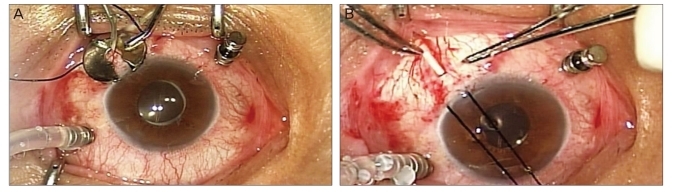
Following retrobulbar anesthesia, a fornix-based conjunctival peritomy with relaxing incisions was made in either the superotemporal or superonasal quadrants, and the adjacent extraocular rectus muscles were isolated with a muscle hook. A Model FP7 Ahmed glaucoma valve (NewWorld Medical Inc., Rancho Cucamonga, CA, USA) was used for six patients and a model FP8 (NewWorld Medical Inc.) for five patients. Patency of the implant tube was verified by irrigation with balanced salt solution. The implant was then anchored 8-10 mm posterior to the limbus between the rectus muscles. A vitrectomy was done using a 23-gauge vitreous cutter driven by a vitrectomy unit and a DORC two-step system (Dutch Ophthalmic Research Company, Exeter, NH, USA). A sclerotomy was placed 3.5 mm posterior to the limbus in all eyes (Fig. 1A). Full panretinal endolaser photocoagulation also was applied if necessary. After the priming of the tube using 1 ml of balanced salt solution, the implant tube was trimmed to a length of 3-4 mm past the neighboring sclerotomy site. Next, the tube was inserted through the cutter probe sclerotomy site and anchored to episclera with 9-0 nylon (Fig. 1B). The implant entry site was covered by a heterologous scleral patch graft. Following removal of the remaining sclerotomy sites, cannula wounds were carefully inspected. Whenever there was wound leakage or a decrease in IOP, a releasable suture was applied with 8-0 nylon followed by closure of the conjunctiva and tenon's capsule. Finally, 10 mg of tobramycin and 1.2 mg of dexamethasone were injected subconjunctivally away from the implant position.
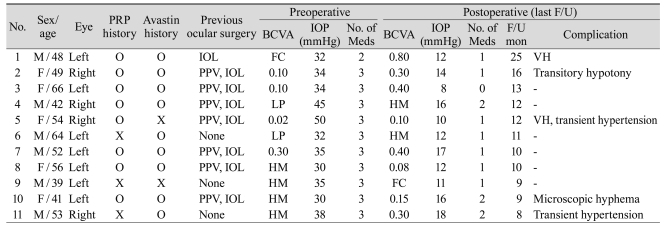
The underlying pathologies were proliferative diabetic retinopathy in all cases. All the patients received at least 6 months of follow-up care. Preoperative laser therapy was not possible due to vitreous hemorrhage or opacity. The angle was able to be checked in only six eyes; they were closed with neovascularization or peripheral anterior synechia. Baseline patient and clinical characteristics are summarized in Table 1.
The mean age of eleven patients (6 male, 5 female) was 51.3 ± 8.8 years (range, 39 to 66 years). The mean follow-up was 12.2 months (range, 8 to 25 months). Eight patients had a previous panretinal photocoagulation (PRP) and seven patients had a previous vitrectomy. Preoperative lens status was pseudophakic in eight eyes and phakic with cataract formation in two eyes which underwent combined phacoemulsification with intraocular lens implantation.
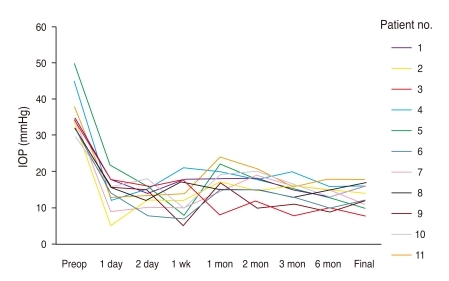
All cases showed significantly improved postoperative IOP. The mean preoperative IOP was 35.9 ± 6.3 mmHg and the average postoperative IOP at the last visit was 13.3 ± 3.2 mmHg. Fig. 2 shows the profile of IOP change within all patients. Even though control of IOP (8 to 18 mmHg) was achieved in all patients, 10 of 11 patients still needed antiglaucoma medication (mean number of medications, 1.2 ± 0.6). The number of topical antiglaucoma medication, however, was reduced from the preoperative 2.9 ± 0.3.
All eleven eyes showed favorable visual acuity with the improvement of mean logarithmically to the minimum angle of resolution visual acuity from preoperative 1.67 ± 0.61 to postoperative 0.96 ± 0.67. In one case (patient number 9), conventional Ahmed valve implantation into the anterior chamber was also performed in the fellow eye because of the same condition in the left eye, which showed an unsatisfactory outcome with the improvement of IOP from 32 mmHg preoperatively to 26 mmHg at final follow up despite using two topical antiglaucoma medications.
No unmanageable intraoperative or postoperative complications developed in this series. A transient hypotony occurred at the first postoperative day in only one eye, which recovered spontaneously. In addition, hypertensive phase occurred in two eyes postoperatively, which responded to the topical antiglaucoma medication. Two eyes of postoperative vitreous hemorrhage and one eye of microscopic hyphema were encountered, but they were stabilized and resolved without any management.
NVG is the final complication of PDR. The incidence of NVG in PDR has significantly declied during the last 10 years due to the improvement in management of diabetic patients and systematic panretinal photocoagulation. However, NVG in PDR classically carries a poor prognosis, typically resulting in severe loss of vision [1].
PRP remains the first-line of therapy for NVG. Secondarily, a filtering procedure associated with antimetabolites or an anterior chamber drainage implant may be used individually if necessary. Bevacizumab has been reported to lead to the regression of neovascularization in eyes with PDR and NVG [14,15].
Drainage devices have improved the management of refractory NVG. Variable success rates for IOP control have been reported ranging from 47% to 96% for Ahmed implant in patients with NVG [16,17]. Typically, drainage tube is implanted through the corneal limbus into anterior chamber. However, in some cases, such as corneal disease and significant neovascularization of the anterior segment, it can be placed through the pars plana into the vitreous cavity.
A drainage implant combined with pars plana vitrectomy has several advantages in PDR patients with uncontrolled IOP. First, media opacities such as vitreous hemorrhage and vitreous opacity may be cleared, facilitating fundus evaluation in the early postoperative period. Second, NVG and PDR may be treated during a single operation. Intraoperative endolaser photocoagulation is helpful in inducing regression of iris neovascularization. Third, pars plana placement of a drainage implant decreases the chance of endothelial touch and hyphema [18]. However, possible posterior segment complications associated with this combined approach include retinal detachment, tube obstruction by vitreous and placement of the tube into the suprachoroidal or subretinal space [18,19]. The advantage of placing the tube into the anterior chamber is that the tube can be visualized directly and tube obstruction can be diagnosed readily [18]. Thus, the decision as to which drainage implant is better when combined with pars plana vitrectomy must be based on the individual clinical situation. In the case of refractory glaucoma with poor anterior chamber condition and vitreoretinopathy, the pars plana implant combined with vitrectomy is usually recommended. In our experience, we prefer the pars plana drainage implant combined with vitrectomy for all refractory NVG in PDR patients regardless of the anterior chamber. We believe that the final visual acuity of the disease is influenced by the progression of the diabetic retinopathy as well IOP.
The vitreous hemorrhage and various cytokines including vascular endothelial growth factor are the main factors associated with the progression of diabetic retinopathy. We hypothesize that pars plana implant may play a role in carrying those factors out of vitreous cavity as well aqueous. Therefore, we propose the drainage implant through the pars plana in PDR with NVG is more effective than the implant of valve through the anterior chamber in PDR progression. In our study, two cases of postoperative vitreous hemorrhages were developed but disappeared within 2 weeks, which is faster than usual. To our knowledge, there are no comparative studies between the anterior chamber and pars plana implant until now.
The complications that occur with implants through anterior chamber include extrusion of the drainage tube, conjunctival erosion with exposure tube, and iritis. Our study did not show any complication associated with tube extrusion, which suggests that the implantation of Ahmed valves through the pars plana can decrease such complications.
We had two hypertensive phases among the 11 cases, which responded to topical medication. In comparison to previous reported rates of 82% [20] and 40% [21], our results indicate a reduced risk of ocular hypertension, and suggest that the combination of 23-gauge pars plana vitrectomy and Ahmed valve implantation is a better surgical approach in eyes with NVG and coexisting vitreoretinal problem such as PDR.
At present, small gauge sutureless vitrectomy is more prevalent than 20-gauge vitrectomy. Pars plana implant could also be applied in small gauge vitrectomy. Recchia et al. [22] first described the technique of small gauge (23 or 25 gauge) vitrectomy combined with glaucoma drainage implant. In that report, primary placement of the tube into the posterior segment was performed in cases of angle closure or corneal endothelial decompensation and was successful in lowering IOP in the majority of patients. Here, we report the outcome of pars plana Ahmed valve implantation combined with 23-gauge vitrectomy in 11 consecutive patients with NVG in PDR. Our experience suggests that the 23-gauge vitrectomy is suitable for pars plana implant because the valve size fits the 23-gauge sclerotomy site.
To our knowledge, an immediate postoperative hypotony may result if the sclerotomy is not closed tightly. The tube lumen of the Ahmed valve fits the 23-gauge needle, which results in no aqueous leakage without additional sutures. It is comparable to the 20-gauge system, which has been associated with an increased risk of postoperative hypotony due to leakage through the incision site [23]. In our study, hypotony-associated complications occurred in only one case (case 2), which was transitory and may be the cause of leakage in the other sutureless incision site. After that, we used a releasable suture in three cases of wound leakage expected in the other slcerotomy site except tube implant site.
The well-known advantages of small gauge vitrectomy such as 23-gauge or 25-gauge over 20-gauge vitrectomy include faster tissue and wound healing, less inflammation, and less patient discomfort [24]. With 23-gauge vitrectomy and pars plana implant surgery compared to the 20 gauge procedure, we also believe that shorter surgery time, faster wound healing, conjunctival preservation, and more patient comfort are convincing factors for its adoption.
In this combined procedure, complete removal of the vitreous at the site of implant is crucial to the success in pars plana implant surgery. For this, we shaved the vitreous base of the cutter site pressing with a depressor. After insertion of the tube through the incision site, we identified the tube tip location in the inner vitreous cavity. In our cases, there is no vitreous strand obstruction.
Presently, all patients showed improved visual acuity and achieved a postoperative IOP between 8 mmHg and 18 mmHg. But 10 of 11 cases still required antiglaucoma medication with at least 1 topical treatment, whose intended usage was to decrease IOP as much as possible because eight of eleven patients were in a last eye state (the other eye is blind).
Even given the small case number, these results are notably consistent compared with previous studies. Due to the small sample size of this study and possible incomparability of cases, it is difficult to conclude the superiority of Ahmed valve implantation into the vitreous cavity combined with 23-gauge vitrectomy. But it is noteworthy that stable and satisfactory results were obtained using 23-gauge and not 23-gauge. No serious postoperative complications were noted in any of the 11 patients during the follow-up period.
Limitations of the current series include its retrospective nature, lack of a control series, small sample size, and limited follow-up period. A direct case-controlled comparison of these procedures and further prospective randomized trials with a longer follow-up will be required to establish the superiority of Ahmed valve implantation combined with 23-gauge pars plana vitrectomy.
In conclusion, the combination of 23-gauge vitrectomy and pars plana Ahmed valve implantation can be an effective and safe surgery in PDR patients with refractory NVG.
Notes
This article was presented as a video in parts at the 102nd autumn meeting of Korean Ophthalmological Society, November 2009, Goyang, Korea.
REFERENCES
1. Shazly TA, Latina MA. Neovascular glaucoma: etiology, diagnosis and prognosis. Semin Ophthalmol 2009;24:113-121.


2. Schocket SS, Nirankari VS, Lakhanpal V, et al. Anterior chamber tube shunt to an encircling band in the treatment of neovascular glaucoma and other refractory glaucomas: a long-term study. Ophthalmology 1985;92:553-562.


3. Lloyd MA, Sedlak T, Heuer DK, et al. Clinical experience with the single-plate Molteno implant in complicated glaucomas: update of a pilot study. Ophthalmology 1992;99:679-687.


4. Hill RA, Heuer DK, Baerveldt G, et al. Molteno implantation for glaucoma in young patients. Ophthalmology 1991;98:1042-1046.


5. Sherwood MB, Smith MF, Driebe WT Jr, et al. Drainage tube implants in the treatment of glaucoma following penetrating keratoplasty. Ophthalmic Surg 1993;24:185-189.


6. Gandham SB, Costa VP, Katz LJ, et al. Aqueous tube-shunt implantation and pars plana vitrectomy in eyes with refractory glaucoma. Am J Ophthalmol 1993;116:189-195.


7. Smiddy WE, Rubsamen PE, Grajewski A. Vitrectomy for pars plana placement of a glaucoma seton. Ophthalmic Surg 1994;25:532-535.


8. Lloyd MA, Heuer DK, Baerveldt G, et al. Combined Molteno implantation and pars plana vitrectomy for neovascular glaucomas. Ophthalmology 1991;98:1401-1405.


9. Luttrull JK, Avery RL. Pars plana implant and vitrectomy for treatment of neovascular glaucoma. Retina 1995;15:379-387.


10. Kaynak S, Tekin NF, Durak I, et al. Pars plana vitrectomy with pars plana tube implantation in eyes with intractable glaucoma. Br J Ophthalmol 1998;82:1377-1382.



11. Sidoti PA, Mosny AY, Ritterband DC, Seedor JA. Pars plana tube insertion of glaucoma drainage implants and penetrating keratoplasty in patients with coexisting glaucoma and corneal disease. Ophthalmology 2001;108:1050-1058.


12. Chalam KV, Gandham S, Gupta S, et al. Pars plana modified Baerveldt implant versus neodymium:YAG cyclophotocoagulation in the management of neovascular glaucoma. Ophthalmic Surg Lasers 2002;33:383-393.


13. Wimpissinger B, Kellner L, Brannath W, et al. 23-Gauge versus 20-gauge system for pars plana vitrectomy: a prospective randomised clinical trial. Br J Ophthalmol 2008;92:1483-1487.


14. Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina 2006;26:352-354.


15. Ciftci S, Sakalar YB, Unlu K, et al. Intravitreal bevacizu mab combined with panretinal photocoagulation in the treatment of open angle neovascular glaucoma. Eur J Ophthalmol 2009;19:1028-1033.


16. Susanna R. Latin American Glaucoma Society Investigators. Partial Tenon's capsule resection with adjunctive mitomycin C in Ahmed glaucoma valve implant surgery. Br J Ophthalmol 2003;87:994-998.



17. Tsai JC, Johnson CC, Dietrich MS. The Ahmed shunt versus the Baerveldt shunt for refractory glaucoma: a single-surgeon comparison of outcome. Ophthalmology 2003;110:1814-1821.


18. Scott IU, Alexandrakis G, Flynn HW Jr, et al. Combined pars plana vitrectomy and glaucoma drainage implant placement for refractory glaucoma. Am J Ophthalmol 2000;129:334-341.


19. Faghihi H, Hajizadeh F, Mohammadi SF, et al. Pars plana Ahmed valve implant and vitrectomy in the management of neovascular glaucoma. Ophthalmic Surg Lasers Imaging 2007;38:292-300.


20. Ayyala RS, Zurakowski D, Smith JA, et al. A clinical study of the Ahmed glaucoma valve implant in advanced glaucoma. Ophthalmology 1998;105:1968-1976.


21. Nouri-Mahdavi K, Caprioli J. Evaluation of the hypertensive phase after insertion of the Ahmed Glaucoma Valve. Am J Ophthalmol 2003;136:1001-1008.


22. Recchia FM, Reichstein DA, Kammer JA. Small-gauge vitrectomy in combination with glaucoma drainage implant procedures. Retina 2010;30:1152-1154.





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




