Clinical Profile and Visual Outcomes after Treatment in Patients with Dysthyroid Optic Neuropathy
Article information
Abstract
Purpose
To report the clinical data and visual outcomes after treatment of patients with dysthyroid optic neuropathy (DON).
Methods
We retrospectively reviewed the medical records and orbital images of 40 patients (65 eyes) with DON and analyzed the visual outcomes after treatment with intravenous steroids pulse therapy, radiotherapy and orbital decompression.
Results
The study included 21 men and 19 women, with 10 (25%) being diabetic patients. Visual field test results revealed defects in 88.7% of DON eyes; afferent pupillary defects in 63.2%; reduced color vision in 78.5%; and abnormal visual evoked potentials in 84%. Orbital imaging showed moderate to severe apical crowding in 95% of the orbits and intracranial fat prolapse in 24.2%. Median best corrected visual acuity improved from 0.4 to 1.0 after one year of treatment (p < 0.001). We noted more improvement in vision with the use of decompressive surgery than with non-surgical methods (p < 0.05). Recurrences occurred in 7 patients who had not received orbital radiotherapy.
Conclusions
Visual field defects and apical crowding seen on orbital imaging were the most sensitive indicators for the detection of DON. Treatment with intravenous steroids pulse therapy, radiotherapy and orbital decompression effectively improved visual outcomes in cases of DON.
Patients with thyroid orbitopathy commonly present with soft tissue features of infiltrative disease, such as eyelid retraction and swelling, conjunctival injection, exophthalmos, and restrictive myopathy; however, in severe cases potentially blinding conditions, such as corneal ulcer and optic neuropathy, may manifest. Dysthyroid optic neuropathy (DON) occurs in approximately 5% of patients with thyroid orbitopathy [1]. DON patients may be asymptomatic with good vision, but it is best assessed by decreased visual acuity, reduced color vision, visual field defects, or afferent pupillary defects [2].
Most cases of DON are considered to occur as the result of optic nerve compression at the orbital apex via expanded intraorbital contents, particularly the extraocular muscles [1,3 9]. The increases in orbital fibroblast synthesis of hyaluronan result in an accumulation of glycosaminoglycan in the retrobulbar tissues [10]. The expansion of retrobulbar tissues in an area of fixed volume increases pressure at the orbital apex, which may inhibit axoplasmic flow [5] or induce optic nerve ischemia [8,11,12].
Treatments widely accepted for patients with DON include systemic corticosteroids, orbital radiation therapy and orbital decompression. Pulse methylprednisolone therapy may provide a rapid means for achieving maximal visual recovery, which can be sustained using a tapering regimen of oral corticosteroids and orbital irradiation [13]. Thyroid orbitopathy is characterized by an autoimmune active inflammation period of 6 to 18 months, during which, treatment with high-dose intravenous corticosteroids may effectively inhibit inflammatory processes and improve visual prognosis [14]. Radiation therapy is also useful in reducing active inflammation with optic nerve compression [5,7,10,15,16]. However, the efficacy of using radiation therapy alone must be determined, as the literature only reports on its utilization in combination with steroid therapy. Some authors have been unable to detect any beneficial effects of radiation therapy when used by itself [17,18]. Bony orbital decompression is considered to be the most effective treatment for DON, in that it provides rapid and persistent relief of apical compression [19].
As there remains controversy over the assessment and management of DON [20], it has proven difficult for clinicians to make an accurate diagnosis and recommend a particular measure to halt the progression of optic neuropathy and restore visual acuity. Asians have shown evidence of less severe orbitopathy than Caucasians, but a rather narrow orbital apex may result in earlier compressive features [2]. The principal objective of this study is to evaluate clinical pictures and treatment outcomes in Korean patients with DON. We have reviewed the clinical data and orbital imaging findings of 40 patients with DON who presented to the Samsung Medical Center, and evaluated the visual outcomes after treatment with intravenous steroids, orbital radiation therapy and/or orbital decompression.
Materials and Methods
We reviewed the records of patients with DON at the Samsung Medical Center from April 1995 to August 2007. Optic neuropathy was diagnosed by one of the following: decreased visual acuity, reduced color vision, visual field defects or relative afferent pupillary defects.
We investigated clinical features, including demographic characteristics, past medical history, thyroid status, as well as its relationship to thyroid orbitopathy. Visual functions were evaluated for best corrected visual acuity, color vision (Ishihara color plates), relative afferent pupillary defect, visual fields (Goldmann or static automated perimetry using the central 30-2 full threshold program), and visual evoked potentials (VEPs). An analysis of orbital imaging (computed tomography or magnetic resonance imaging) was conducted to document the compression of the optic nerve at the orbital apex and orbital volume expansion. We evaluated visual outcomes and recurrence after treatment.
Orbital imaging parameters included optic nerve crowding, muscle index, and intracranial fat prolapse. Optic nerve crowding induced by enlarged extraocular muscles at the orbital apex was assessed on the coronal images using the grading scale described by Nugent et al. [21]. Grade 0 reflects no effacement of the perineural fat planes due to enlarged extraocular muscles, grade 1 (mild) reflects 1% to 25% effacement, grade 2 (moderate) reflects 25% to 50% effacement and grade 3 (severe) reflects effacement greater than 50%. Muscle indices (MIs) were calculated in accordance with the method described by Barrett et al. [22]. Measurements were obtained from coronal scans at a point halfway between the posterior globe and the orbital apex. The transverse dimensions of the horizontal muscle groups (medial and lateral recti) and the orbital width were measured along a horizontal line through the optic nerve. The horizontal MI was expressed as the percentage of orbital width occupied by the medial and lateral rectus muscles. The vertical MI was expressed as the percentage of the height of the orbit occupied by the inferior rectus and the superior muscle group. The greater of these two values was taken as the final muscle index. Intracranial fat prolapse was determined using Birchall et al.'s method [23]. The extent of posterior orbital fat was defined relative to the boundaries of the superior ophthalmic fissure, which were defined on the axial images obtained, parallel to the Reid baseline. The lateral margin of the superior ophthalmic fissure was defined as the most medial portion of the inner border of the sphenoid wing. The medial margin was identified as the anterior border of the groove in the sphenoid body formed by the terminal intracavernous portion of the internal carotid artery. Intracranial fat prolapse was considered to be present if fat was detected 2 mm or more posterior to this line.
Treatment options consisted of one of the following three modalities: intravenous methylprednisolone pulse (IVMP) therapy, orbital radiation therapy and orbital decompression (bony removal of the medial and inferior wall). Methylprednisolone (250 mg) was administered every six hours via intravenous infusion for three consecutive days. Patients failing IVMP therapy underwent orbital decompression via transcaruncular or transnasal endoscopic approaches. After the completion of intravenous corticosteroids or decompression surgery, 80 mg of prednisone was orally administered for seven days and tapered at 10 mg a week until a dose of 20 mg was reached, then tapered by 5 mg every week. Orbital radiation therapy was instituted in patients whose optic neuropathy recurred or when orbital inflammation persisted, despite treatment with corticosteroids or orbital decompression. The orbital radiation dose was 20 Gy in 10 fractions over two weeks.
Descriptive statistics were utilized in order to describe the basic features of the data in our study. We assessed changes in visual acuity after treatment using the paired t-test. An independent t-test and the Mann-Whitney test were utilized in order to compare visual acuity changes in the orbital decompression group versus those in the non-decompression group. Decimal visual acuity data were converted logarithmically to the minimum angle of resolution (logMAR) equivalents for statistical analysis. Statistical significance was indicated when p < 0.05.
Results
Sixty-five eyes of 40 patients (21 male, 19 female) were included in this study. Twenty-five patients had bilateral DON and 15 patients had unilateral optic neuropathy. The average age at the time of diagnosis was 50.0 years (range, 20 to 74 years). We noted no age difference between sexes. It required a median 5.5 months (range, 0 days to 120 months) for the patients to develop DON after the onset of thyroid eye disease, and the interval between subjective symptoms of DON and the first therapy was a median of 2 months (range, 7 days to 11 months). The median follow-up time after the initial treatment was 18 months (range, 5 to 120 months).
The results of the thyroid function test showed that 32 patients (80%) were euthyroid, 4 (10%) were hyperthyroid and 4 (10%) were hypothyroid. Ten patients (25%) also had type II diabetes mellitus.
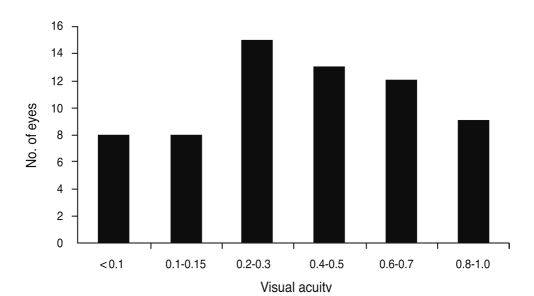
The median best corrected visual acuity of the 65 eyes with DON at presentation was 0.4 (range, hand motion to 1.0) (Fig. 1). The visual field was abnormal in 55 eyes (88.7%) out of 62 eyes for which data were available. Color vision was abnormal in 51 eyes (78.5%) out of 65 ey es with DON, whose mean Ishihara color plate values were 6.7 (range, 0 to 15). Relative afferent pupillary defects were noted in 24 patients (63.2%) out of the 38 tested. VEPs were assessed in 30 patients. Of 50 eyes with DON, the VEPs were determined to be abnormal in 42 eyes (84.0%), with 37 having delayed latency and 21 with decreased amplitude. The results of fundus examination were obtained in 55 DON eyes: normal in 29 eyes (52.7%), disc edema in 23 eyes (41.8%), and optic atrophy in 3 eyes (5.5%).
We were able to review the orbital imaging of 62 orbits with DON (Table 1). Grade 3 (severe) optic nerve crowding was noted in 32 (51.7%) orbits. No orbits with DON evidenced less than grade 1 apical crowding. A muscle index of 67% or greater was noted in 28 eyes (45.2%). None of the DON orbits had muscle indices of less than 50%. Intracranial fat prolapse was noted in 15 orbits (24.2%).
Treatment for DON consisted of a combination of three modalities (Table 2). IVMP therapy was utilized in the initial management of 39 patients with DON: 6 patients were treated successfully by corticosteroids alone; 16 patients who failed to improve on corticosteroids subsequently received orbital decompression; and 25 patients were treated with radiation therapy. One untreated patient had normal visual acuity, but also had an abnormal visual field and a color perception defect. She refused any treatment, and her visual field and color vision returned to normal without treatment after six months.
Average visual acuity improved from a median of 0.4 to 0.7 one month after the initial treatment, no change was seen in the subsequent five months, visual acuity reached 0.9 at 12 months and 1.0 at 24 months. Of the 54 eyes of 32 patients, available for evaluation for a 12-month follow-up visit, all showed improvements in visual acuity by 2 lines or more. The delta logMAR was statistically significant during the first month (p < 0.001) and between 6 and 12 months (p < 0.05) following treatment initiation (Fig. 2).
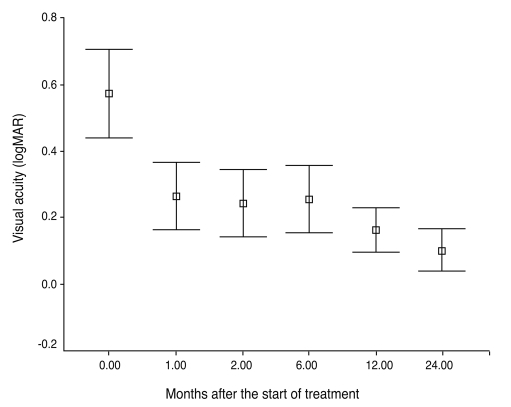
Change of logarithmically to the minimum angle of resolution (logMAR) visual acuity from time of diagnosis to 2 years after the start of treatment. Improvement of vision was statistically significant during the initial month (p < 0.001) and between 6 and 12 months (p < 0.05) after the initiation of treatment.
The patients were divided into a decompression group (26 eyes of 16 patients) and a non-decompression group (39 eyes of 24 patients) (Fig. 3). While baseline logMAR visual acuity was 0.85 ± 0.70 in the decompression group and 0.38 ± 0.28 in the non-decompression group (p = 0.002), we noted no significant differences between the two groups after six months. Thus, orbital decompression achieved a greater improvement of vision than non-surgical treatment (p < 0.05).
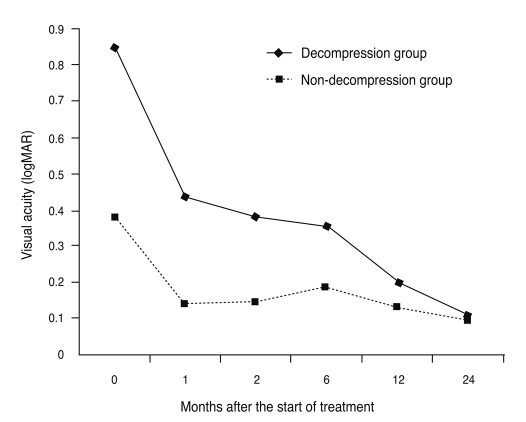
Comparison of change in average visual acuity between the decompression group (26 eyes of 16 patients) and the non-decompression group (39 eyes of 24 patients). logMAR = logarithmically to the minimum angle of resolution.
Recurrences occurred in 7 patients: 4 patients with IVMP therapy alone and 3 patients with IVMP and orbital decompression. None of the 20 patients who had received radiation therapy experienced a recurrence, compared with 7 of the 14 patients treated without radiation therapy (p = 0.001). It required a mean of 3.8 months (median 4 months; range, 1 to 7 months) to develop recurrent neuropathy following IVMP and/or orbital decompression. All of the patients who had a recurrence recovered fully after treatment with IVMP or radiation therapy.
Discussion
DON is derived from thyroid orbitopathy, but is different in terms of its epidemiological features [1]. The complexity of DON and the variability of the presenting symptoms and signs of this condition present a challenge to clinicians who wish to develop a standardized clinical assessment [20]. As there are differences in the degree of thyroid orbitopathy and orbital anatomy between Caucasians and Asians, we must evaluate the clinical profile and visual outcomes of Asian patients with DON. We assessed 40 Korean patients treated for DON at the Samsung Medical Center between 1995 and 2007. Management was individualized for each patient's DON condition.
Thyroid orbitopathy generally affects women in their fourth and fifth decades, the female-to-male ratio being approximately 4:1 [1]. Women are up to eight times more likely to evidence severe orbital involvement [10]. DON typically manifests when a person is between 40 and 80 years old, and there is no prominent gender predilection; the female-to-male ratio has been reported as 1.6 to 2.4:1 [3,4,20]. Patients with DON tend to be 8 to 11 years older at the time of examination than the patients without DON [3,4]. The sex ratio of our cases was almost 1:1, which corresponds to a higher incidence of DON in males than has been noted in previous studies. This indicates that males are much more likely to develop optic neuropathy than are female patients with thyroid orbitopathy, and that severe orbital involvement of thyroid disease does not necessarily result in neuropathy. We had no pediatric patients (under the age of 18 years), as has also been the case in past reports [24-26]. It appears that patients younger than 20 years old are not likely to evidence severe infiltration of the extraocular muscles.
Diabetes was noted in 25% of our cases, which supports the notion that vasculopathy is one of the pathogenetic factors of DON. Marginal oxygenation due to preexisting vasculopathy may make the optic nerve more susceptible to ischemic changes because of the compression [27]. Diabetics are believed to suffer from a more impetuous and more recalcitrant course, and tend to evidence poorer final visual results [3,28]. Seven out of 10 diabetic patients in this study required decompressive surgery with poor response to corticosteroids, but they achieved the same final visual acuity as did the non-diabetics. Diabetes is a risk of DON, but it does not influence visual outcomes when neuropathy is treated appropriately.
The symptoms of DON manifested at a median of 5.5 months after the onset of eye symptoms of thyroid orbitopathy, with 5 cases of neuropathy developing with thyroid eye disease simultaneously. In the active inflammatory phase of thyroid orbitopathy, optic nerve compression by the retrobulbar tissue swelling renders optic neuropathy much more likely to occur. Decreased visual acuity was the most common complaint of our patients at the initial presentation. Pretreatment visual acuity was a median of 0.4, and the severity of visual loss was similar to that described in other reports [3,4,20]. Other chief complaints included aching ocular sensation, abnormal color perception, and visual field disturbances. Some patients did not even realize they were experiencing symptoms of optic nerve dysfunction. The authors suggest that careful history taking and thorough physical examination should be conducted to detect this significant complication, particularly during the early inflammatory phase of thyroid orbitopathy.
Studies have demonstrated that computed tomography scanning provides objective measures that can be used to identify patients who have an increased risk of concurrent dysthyroid optic neuropathy [3,7,20-23]. MI, optic nerve crowding and intracranial fat prolapse were shown to be significantly related to the presence of DON in the literature, and thus were adopted as measures for the quantification of optic nerve compression and orbital tissue volume increases in this study.
Apical crowding has been reported to be a highly sensitive and specific indicator of neuropathy [7,21,23]. We noted grade 2 and 3 apical crowding in 59 (95.2%) DON orbits. Grade 1 (mild) apical crowding was also noted in 3 (4.8%) orbits, which clearly demonstrated clinical features of optic neuropathy and responded well to IVMP and radiation therapy. In the absence of apical compression by pathologically enlarged extraocular muscles, increased orbital pressure may have induced DON [29]. With regard to the MI, it was previously reported that an MI <50% was able to exclude DON but an MI above 67% was not in unanimous agreement in excluding the negative cases [7,22]. Our data revealed that all DON orbits had an MI >50%, and approximately half of them had an MI >67%. It appears that an MI >50% is very sensitive for DON, and should be considered a risk for compressive optic neuropathy. Intracranial fat prolapse was described as an indicator of DON with a sensitivity of 75% to 94% and a specificity of 52.9% to 91% [7,23]. We noted intracranial fat prolapse in only 24.2% of the cases, a similar rate to that noted in a recent study [20]. Therefore, it would appear to have less value in detecting DON on computed tomography than has been previously suggested.
Moderate to severe apical crowding and an MI >50% are believed to be sensitive parameters indicating DON, but they cannot directly demonstrate the degree of optic nerve damage, such that their presence indicates optic nerve function tests and close observation are necessary for selecting the optimal management strategy.
In the management of DON, we employed IVMP as the initial therapeutic trial. The intravenous route delivers a higher cumulative dose of corticosteroids with fewer side effects, and is considered to be more effective and better tolerated than the oral route [2,14]. Previous report revealed improvement rates of 39% to 100%, and remissions were maintained with oral prednisone and orbital irradiation [13,14,30]. Our results revealed that 23 (59.0%) patients were stabilized with non-surgical treatment, including corticosteroids and radiotherapy. Among these, six patients responded to corticosteroids and remained stable without additional therapy, and 17 patients required adjunctive radiation therapy. Sixteen (41.0%) patients received subsequent decompressive surgery as symptoms of DON did not improve or relapsed immediately after IVMP.
Patients in the surgery group evidenced significantly worse visual acuity at diagnosis than those in the non-surgical group (logMAR 0.85 ± 0.70 vs. 0.38 ± 0.28). We can infer that patients with poor visual acuity are less inclined to respond to IVMP, and will require bony decompression. Patients in the surgery group evidenced rapid improvement in optic nerve functions after decompression, and eventually attained a final visual acuity equal to that of the patients treated without decompression. This lends credence to two points: first, bony apex decompression is the most effective treatment for DON [19], and secondly, the delay of orbital decompression by first trying IVMP does not affect the ultimate visual function [30]. Although bony apex decompression continues to be a definitive treatment for DON [1,19,31-34], surgery may involve associated risks and may not be readily conducted in many medical facilities. We believe that good assessments should be made and management should be individualized to induce maximal benefits, thus reducing associated harms.
Radiotherapy is useful in actively inflamed orbits with optic nerve compression [1,5,10,15,16]. Combination therapy with high-dose steroids proved more effective due to the rapid action of steroids during the short-term period and the more delayed (but sustained) action of radiation [14,31,32]. However, no studies thus far have assessed the effects of radiotherapy as the sole therapeutic modality for DON without adjunctive therapy, and some authors have denied its having beneficial therapeutic effects in thyroid orbitopathy [17,18]. It is our impression that radiotherapy alone does not affect improvements in optic nerve functions, but its sustained anti-inflammatory effects prevent the recurrence of neuropathy following the immediate recovery of DON after treatment with IVMP or decompression surgery.
All of our patients evidenced improvement in visual acuity by two lines or more after treatment. Visual acuity evidenced marked improvement within one month after the onset of therapy, remained stable for five months, improved again in the subsequent six months, and then stabilized thereafter. Initial improvements in visual acuity are probably related to the relief of optic nerve compression in the orbital apex and reductions in optic nerve perfusion as the result of decompressive surgery and steroids. It appears unlikely that these two therapeutic modalities continue to be associated with improvement more than six months after treatment, as visual acuity remained stable for five months after immediate recovery. Radiotherapy appears not to affect the later recovery process directly, because visual acuity was the same whether or not the patients received radiotherapy. Carter et al. [33] previously stated that eyes which required weeks to months prior to evidencing maximum recovery may have had more fibrotic ocular muscles or orbital tissue compressing the optic nerve. Later improvement of visual acuity may be explained by lessened thyroid eye disease activity and visual recovery following treatment for recurring neuropathy.
Although we noted one patient whose reduced color vision and abnormal visual field returned to normal without specific treatment, the natural course of DON is difficult to predict, and is certainly not benign [1]. We have noted relatively good visual outcomes with adequately combined corticosteroid, radiotherapy and orbital decompression therapeutic modalities. Visual acuity continued to improve for up to one year after treatment. Physicians should seek individualized therapy, weighing the benefits and risks of each modality. After successful primary therapy, a close follow-up evaluation of the patient should be made when oral prednisone is tapered or discontinued, as recurrences may readily occur during this period. Radiotherapy can be routinely administered to all patients without contraindications after the immediate recovery in order to reduce recurrence.
Notes
No potential conflict of interest relevant to this article was reported.
Presented at the American Society of Ophthalmic Plastic and Reconstructive Surgery 40th anniversary fall scientific symposium, San Francisco, October 21-22, 2009.