 |
 |
| Korean J Ophthalmol > Volume 38(2); 2024 > Article |
|
Abstract
Purpose
To evaluate the refractive outcomes after ultrathin Descemet stripping automated endothelial keratoplasty (UT-DSAEK) combined with phacoemulsification and intraocular lens implantation (triple procedure) in the South Korean population.
Methods
This retrospective observational study included 37 eyes of 36 patients who underwent the UT-DSAEK triple procedure between 2012 and 2021 in a single tertiary hospital. Preoperative and postoperative refractive outcomes and endothelial parameters at 1, 3, 6, and 12 months were observed.
Results
At the final postoperative 12-month period, the average best-corrected visual acuity was 0.4 ± 0.5 in logarithm of the minimum angle of resolution. The mean endothelial cell density at 12 months was 1,841.92 ± 731.24 cells/mm2, indicating no significant endothelial cell loss compared to the baseline (p = 0.128). The mean postoperative central corneal thickness at 12 months was 597.41 ± 86.26 μm. The postoperative mean absolute error at 12 months was 0.96 ± 0.89 diopters (D) and mean error was 0.89 ± 0.97 D.
Corneal transplantation is one of the most commonly performed allogenic transplants globally, serving as a primary treatment for various corneal endothelial dysfunctions, such as Fuchs dystrophy and bullous keratopathy. Endothelial keratoplasty, particularly Descemet stripping automated endothelial keratoplasty (DSAEK) and Descemet membrane endothelial keratoplasty (DMEK), has gained preference over penetrating keratoplasty (PK) in recent years [1,2]. Unlike PK, which involves the restoration of the entire corneal layer, DSAEK entails the transplantation of the posterior stroma, Descemet membrane, and the endothelium. DMEK involves the removal and transplantation of Descemet membrane and the endothelium with a smaller incision and fewer stitches, resulting in advantages, such as rapid visual recovery and high predictability in postoperative keratometry [3-6]. However, DMEK requires a more challenging procedure in complicated eyes due to the thin thickness and fragility of the corneal graft [7,8]. Ultrathin DSAEK (UT-DSAEK) utilizes thinner donor grafts ranging from less than 80 to 100 μm [9] and shows excellent visual outcomes comparable to DMEK [10].
As patients with corneal endothelial dysfunctions suffer from coexisting cataracts, cataract surgery is often performed concurrently (triple procedure) with keratoplasty [11]. Previous studies indicate that refractive shift ranges from +0.31 ± 2.03 to +1.26 ± 0.53 diopters (D) after DSAEK and from −1.14 ± 1.7 to +0.90 ± 1.5 D after DMEK [12-17]. Predicting refractive outcomes is particularly challenging in the presence of corneal edema after endothelial keratoplasty, emphasizing its importance in selecting the appropriate power of the intraocular lens (IOL) in combined surgeries.
Despite numerous studies reporting on the clinical outcomes of the UT-DSAEK triple procedure, no studies have specifically focused on the South Korean population. Therefore, the purpose of this study is to determine refractive outcomes and errors after UT-DSAEK combined with cataract extraction and IOL implantation in the South Korean population.
The study protocol was reviewed and approved by the Institutional Review Board of Seoul St. Mary’s Hospital (No. KC22RASI0043). The study was performed in accordance with the tenets of the Declaration of Helsinki. The requirement for informed consent was waived due to the retrospective nature of the study.
This retrospective observational study reviewed 48 eyes of 46 patients, who underwent the UT-DSAEK triple procedure from January 2012 to September 2021 at the Seoul St. Mary’s Hospital (Seoul, Korea).
We analyzed patients, who underwent UT-DSAEK triple procedures due to clinically significant cataract with corneal endothelial dysfunction. Exclusion criteria were as follows: (1) intraoperative complications of cataract surgery (i.e., posterior capsule rupture, vitreous prolapse, or IOL instability due to zonulysis, conversion to extracapsular cataract extraction and IOL scleral fixation); (2) the presence of underlying comorbidities that impair vision, including uncontrolled or late-stage glaucoma, retinal disorders, optic neuropathy, and more; and (3) primary graft failure (i.e., failure to clear edematous condition at any time within 3 months postsurgery). Regarding the exclusion criteria mentioned above, a total of 37 eyes of 36 patients were included in this study.
Preoperative, intraoperative, and postoperative data were collected to assess the measurements. The final observation was conducted 1 year postoperatively. The main measures included the recipient’s best-corrected visual acuity (BCVA); preoperative anterior chamber depth (in millimeters) and axial length (in millimeters) by optical coherence tomography-based biometry (IOLMaster 700, Carl Zeiss Meditec AG); refractive spherical equivalent (SE; in diopters); corneal curvature; flat keratometry (K1), steep keratometry (K2), keratometry average (Kavg), and corneal astimgmatism (Kast) by automated keratometry (KR-1, Topcon); endothelial cell density (ECD; in cells/mm2) by specular microscopy (CellChek SL, Konan); and central corneal thickness (CCT; in micrometers) by ultrasonic pachymeter (SP-3000, Tomey). Donor cornea graft thickness, diameters, ECD, and duration of death to cooling, death to preservation time, and preservation to surgery time for precut tissue and surgeon cut tissue were observed. The duration from death to cooling for surgeon cut tissue was not analyzed as this information was unavailable due to the retrospective nature of our study. BCVA was assessed using a Snellen eye chart (decimal) and converted into logarithm of the minimum angle of resolution (logMAR). Hand motion and finger count were assigned logMAR values of 2.5 and 1.9, respectively. Based on previous reports [12-17], the IOL target diopter was set −0.75 D myopic to the target diopter using the Barrett Universal II IOL formula, regarding the hyperopic shift effect of the DSAEK graft. The mean error (ME) and the mean absolute error (MAE) were evaluated at 1, 3, 6, and 12 months after surgery. ME was defined as the difference between predicted and postoperative SE refraction, while MAE was defined as the absolute difference between predicted and achieved postoperative SE refraction.
All operations were performed under general anesthesia by four surgeons (HSK, CKJ, SHC, YSB). All donor tissues were stored in corneal storage solution (Optisol, Bausch & Lomb Surgical). The preparation of DSAEK lenticules utilized either precut tissue or surgeon cut tissue. For the surgeon cut method, domestic cornea was utilized given from the Eye Bank of Korea at Seoul St. Mary’s Hospital, while precut tissue was prepared by the imported cornea from Eversight Eye Bank (Chicago, IL, USA).
The surgeon cut method was as follows. Briefly, the donor cornea was mounted on an artificial anterior chamber (Moria Surgical) with an infusion bottle. Then, the corneal epithelium was debrided, and graft was dissected using a microkeratome (Moria Surgical) with a 400-micron microkeratome head. The anterior cap was lifted, and residual stromal bed was measured using a contact pachymetry. Manual dissection of the peripheral anterior stromal lamella was performed.
In all cases, the UT-DSAEK triple procedure was executed in combination of the pull-through insertion technique [18] using the EndoGlide (Network Medical Products) and cataract surgery (phacoemulsification and IOL insertion). In brief, a 4.5-mm scleral tunnel was made followed by a 2.2-mm corneal entrance incision, and the anterior chamber was filled with viscoelastics. A continuous curvilinear capsulorhexis, hydrodissection, and phacoemulsification were performed consecutively. After inserting a foldable IOL into the capsular bag, the recipient’s Descemet membrane was removed by using the reverse Sinsky hook. Miochol (Novartis AG) was injected into the anterior chamber, and inferior iridotomy was performed to prevent pupillary block. A 1-mm incision site was made 180° counterpart from the main incision for the curved microforcep to pass through. An anterior chamber maintainer was introduced into the side incision site to prevent anterior chamber collapse during insertion, and the remaining viscoelastics in anterior chamber were removed by an irrigation/aspiration device. The main incision site was further extended to a width of 4.5 mm. The prepared donor graft was loaded in the EndoGlide, then inserted into the anterior chamber using forceps from the counterpart. Once the graft was positioned properly, all incisions were sealed with 10-0 polyprolene sutures, and sterile air was injected to achieve a thorough attachment of the graft to the recipient’s stoma.
Statistical analysis was performed by using IBM SPSS ver. 23.0.1 (IBM Corp). The data were presented as mean ± standard deviation. Wilcoxon signed rank test was used to compare preoperative and postoperative ocular parameters. Repeated Measures analysis of variance was used to identify the change of postoperative refractive errors. A p-value of less than 0.05 was considered statistically significant.
The characteristics of 37 eyes of 36 patients are summarized in Table 1. The most common diagnosis for UT-DSAEK triple procedure in this study was Fuchs dystrophy (15 eyes, 40.6%). The preoperative biometric data are summarized in Table 2. The preoperative ECD was 1,247.11 ± 609.44 cells/mm2 and the preoperative average CCT was 735.75 ± 127.39 μm. Donor graft data are summarized in Table 3. The mean donor graft ECD was 2,909.08 ± 313.18 cells/mm2. The mean donor corneal graft thickness for precut tissue was 57.84 ± 12.24 μm and the mean graft diameter was 7.87 ± 0.21 μm, while the mean corneal graft thickness for surgeon cut tissue was 67.40 ± 7.54 μm and the mean graft diameter was 8.05 ± 0.21 μm. The mean duration between death to cooling time, death to preservation time, and preservation to surgery were measured as 3.33 ± 1.29, 7.45 ± 4.07, and 127.04 ± 39.29 hours, respectively, for precut tissue; and death to preservation time and preservation to surgery were measured as 2.06 ± 1.90 and 72.21 ± 43.19 hours, respectively, for surgeon-cut tissue. Due to some patients with slow recovery, it was impossible to acquire precise measurements, including ECD and keratometry, and only 29 eyes were analyzed at the final 12-month period. BCVA after UT-DSAEK triple procedure is shown in Table 4 and Fig. 1. BCVA improved from preoperative BCVA logMAR 1.35 ± 0.61 to logMAR 0.7 ± 0.4 at postoperative 1 month, logMAR 0.6 ± 0.4 at 3 months, logMAR 0.5 ± 0.4 at 6 months, and logMAR 0.4 ± 0.5 at 12 months, with significance (p < 0.001). The postoperative SE was 0.29 ± 3.01 D at postoperative 1 month, 0.05 ± 1.54 D at 3 months, −0.47 ± 1.06 D at 6 months, and −0.57 ± 1.06 D at 12 months. Mean ECD was 1,839.00 ± 659.81 cells/mm2 at postoperative 3 months (p = 0.080) and 1,841.92 ± 731.24 cells/mm2 at 12 months (p = 0.128), which had no significant change compared to the donor cornea. Postoperative Kavg was 43.68 ± 1.38 D at final observation and showed no significant change compared to the preoperative value (p = 0.276). Mean CCT showed significant change since postoperative 3-month period: 613.25 ± 93.05 μm at postoperative 3 months (p = 0.004), 584.75 ± 48.57 μm at 6 months (p = 0.011), and 597.41 ± 86.26 μm at 12 months (p = 0.001). CCT measurements at every postoperative time point showed significant decrease compared to the preoperative value.
To our knowledge, this is the first study to report the clinical results of the UT-DSAEK concurrently combined with cataract surgery in the South Korean population. The present study assessed the refractive outcome up to 1 year following the UT-DSAEK triple procedure. As previously noted, hyperopic outcomes are common after DSAEK surgery, due to differences between the central and peripheral thicknesses of the donor graft [15,19]. Additional potential contributors to the postoperative hyperopic shift include corneal edema swelling, leading to anterior curvature flattening and posterior curvature steepening [20]. Therefore, surgeons often aim for a more myopic postoperative outcome by targeting a postoperative refraction of −1.00 to −2.00 D [5,12,14,21,22]. In our study, we selected the IOL diopter with −0.75 D myopic target [23]. Nevertheless, the postoperative SE error at 12 months was −0.57 ± 1.06 D, leading to ME of 0.89 ± 0.97 D. This result agrees with previous reports, considering cases of corneal decompensation that can result in undesirable hyperopic refraction [11]. When observing MAE, there was a gradual reduction in errors deviating from the target, and in terms of ME, it tended to become less hyperopic over time. This postoperative change seems to be due to the combined effect of reduction in corneal edema and IOL stability in the capsular bag. There were previous reports of correlation between graft thickness and hyperopic change. As reported by Jun et al. [15], there was a small negative correlation between the refractive change and graft thickness, with no significance. (r = −0.16, p = 0.31). Aligning with previous results, also in our study, there was no significant correlation between precut thickness and MAE (r = −0.341, p = 0.154) nor ME (r = −0.388, p = 0.101) throughout the period. Also as reported by Yoo et al. [19], they compared the central to peripheral graft thickness ratio and hyperopic effect rather than assessing the graft thickness, which indicates that graft thickness itself is not the determinant factor of refractive change. Our study included five cases of manual surgeon cut graft. However, the graft thickness did not show significant difference between the precut tissue and surgeon cut tissue (57.84 ± 12.24 μm vs. 67.40 ± 7.54 μm, p = 0.678; not shown in this article).
There has been a debate on whether the simultaneous UT-DSAEK triple procedure or sequential procedure yields better outcomes. Some argue that sequential, nonsimultaneous endothelial keratoplasty, cataract extraction, and IOL implantation surgery provides superior final refractive results compared to the triple procedure because the accuracy of determining IOL power is higher on the basis of postoperative keratometry [24]. However, there was no statistically significant difference in visual outcome and refractive error in patients who underwent either triple or sequential procedures [25,26]. Although postoperative complications such as corneal edema can reduce the accuracy of refractive outcomes in simultaneous UT-DSAEK triple procedure, the greatest advantage of the combined triple procedure lies in its ability to minimize potential surgical risks, particularly in older patients, and alleviate the additional cost associated with a second procedure.
As widely acknowledged, DMEK exhibits favorable outcomes, but it demands more advanced surgical skills. In situations where patients exhibit advanced corneal edema or have an unstable posterior capsule, the complexities of the DMEK procedure are heightened, making UT-DSAEK a preferred choice for its safer and more favorable outcomes [27,28].
This study has several limitations. First, since this study is not a case-control or comparative cohort study, we cannot draw conclusions regarding the differences between the triple and sequential procedures. Furthermore, as reported by Clemmensen et al. [29], the hyperopic shift was partially due to the changes in the anterior and posterior surface curvatures of the cornea, which was not analyzed in our study. Despite these limitations, our study strengthens the fact that it is the first investigation of refractive outcomes of UT-DSAEK triple in the Korean population.
In conclusion, UT-DSAEK triple procedure showed favorable and safe outcomes, with consistency in refractive changes in the Korean populations, which corresponds to previous studies.
Acknowledgements
The authors would like to acknowl edge Choun-Ki Joo (CK St. Mary’s Eye Clinic, Seoul, Korea) for his role in performing the operations.
References
1. Melles GR, Eggink FA, Lander F, et al. A surgical technique for posterior lamellar keratoplasty. Cornea 1998;17:618-26.


2. Singh R, Gupta N, Vanathi M, Tandon R. Corneal transplantation in the modern era. Indian J Med Res 2019;150:7-22.



4. Koenig SB, Covert DJ. Early results of small-incision Descemet’s stripping and automated endothelial keratoplasty. Ophthalmology 2007;114:221-6.


5. Lee WB, Jacobs DS, Musch DC, et al. Descemet’s stripping endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology 2009;116:1818-30.


6. Stuart AJ, Romano V, Virgili G, Shortt AJ. Descemet’s membrane endothelial keratoplasty (DMEK) versus Descemet’s stripping automated endothelial keratoplasty (DSAEK) for corneal endothelial failure. Cochrane Database Syst Rev 2018;6:CD012097.


7. Deng SX, Lee WB, Hammersmith KM, et al. Descemet membrane endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology 2018;125:295-310.


8. Singh A, Zarei-Ghanavati M, Avadhanam V, Liu C. Systematic review and meta-analysis of clinical outcomes of descemet membrane endothelial keratoplasty versus Descemet stripping endothelial keratoplasty/descemet stripping automated endothelial keratoplasty. Cornea 2017;36:1437-43.


9. Busin M, Patel AK, Scorcia V, Ponzin D. Microkeratome-assisted preparation of ultrathin grafts for Descemet stripping automated endothelial keratoplasty. Invest Ophthalmol Vis Sci 2012;53:521-4.


10. Dunker SL, Dickman MM, Wisse RPL, et al. Descemet membrane endothelial keratoplasty versus ultrathin Descemet stripping automated endothelial keratoplasty: a multicenter randomized controlled clinical trial. Ophthalmology 2020;127:1152-9.


11. Covert DJ, Koenig SB. New triple procedure: Descemet’s stripping and automated endothelial keratoplasty combined with phacoemulsification and intraocular lens implantation. Ophthalmology 2007;114:1272-7.


12. Dupps WJ Jr, Qian Y, Meisler DM. Multivariate model of refractive shift in Descemet-stripping automated endothelial keratoplasty. J Cataract Refract Surg 2008;34:578-84.



13. Ham L, Dapena I, Moutsouris K, et al. Refractive change and stability after Descemet membrane endothelial keratoplasty: effect of corneal dehydration-induced hyperopic shift on intraocular lens power calculation. J Cataract Refract Surg 2011;37:1455-64.

14. Holz HA, Meyer JJ, Espandar L, et al. Corneal profile analysis after Descemet stripping endothelial keratoplasty and its relationship to postoperative hyperopic shift. J Cataract Refract Surg 2008;34:211-4.


15. Jun B, Kuo AN, Afshari NA, et al. Refractive change after Descemet stripping automated endothelial keratoplasty surgery and its correlation with graft thickness and diameter. Cornea 2009;28:19-23.


16. van Dijk K, Ham L, Tse WH, et al. Near complete visual recovery and refractive stability in modern corneal transplantation: Descemet membrane endothelial keratoplasty (DMEK). Cont Lens Anterior Eye 2013;36:13-21.


17. van Dijk K, Rodriguez-Calvo-de-Mora M, van Esch H, et al. Two-year refractive outcomes after Descemet membrane endothelial keratoplasty. Cornea 2016;35:1548-55.


18. Sarnicola V, Millacci C, Sarnicola E, et al. Suture pull-through insertion of graft donor in Descemet stripping automated endothelial keratoplasty: results of 4-year follow-up. Taiwan J Ophthalmol 2015;5:114-9.



19. Yoo SH, Kymionis GD, Deobhakta AA, et al. One-year results and anterior segment optical coherence tomography findings of Descemet stripping automated endothelial keratoplasty combined with phacoemulsification. Arch Ophthalmol 2008;126:1052-5.


20. Chamberlain W, Shen E, Werner S, et al. Changes in corneal power up to 2 years after endothelial keratoplasty: results from the randomized controlled Descemet endothelial thickness comparison trial. Am J Ophthalmol 2023;245:233-41.


21. Terry MA, Shamie N, Chen ES, et al. Endothelial keratoplasty for Fuchs’ dystrophy with cataract: complications and clinical results with the new triple procedure. Ophthalmology 2009;116:631-9.


22. Schoenberg ED, Price FW Jr, Miller J, et al. Refractive outcomes of Descemet membrane endothelial keratoplasty triple procedures (combined with cataract surgery). J Cataract Refract Surg 2015;41:1182-9.


23. Laaser K, Bachmann BO, Horn FK, et al. Descemet membrane endothelial keratoplasty combined with phacoemulsification and intraocular lens implantation: advanced triple procedure. Am J Ophthalmol 2012;154:47-55.


24. Geggel HS. Intraocular lens implantation after penetrating keratoplasty: improved unaided visual acuity, astigmatism, and safety in patients with combined corneal disease and cataract. Ophthalmology 1990;97:1460-7.


25. Ozbek Uzman S, Yalniz Akkaya Z, Duzova E, et al. Corneal pathology and cataract: combined surgery or sequential surgery? Turk J Ophthalmol 2021;51:1-6.



26. Pineros OE, Cohen EJ, Rapuano CJ, Laibson PR. Triple vs nonsimultaneous procedures in Fuchs’ dystrophy and cataract. Arch Ophthalmol 1996;114:525-8.


27. Han YE, Chung HS, Lee H, et al. Clinical outcomes of nanothin Descemet stripping automated endothelial keratoplasty in Korean patients with corneal endothelial dysfunction. Korean J Ophthalmol 2022;36:131-7.




Fig. 1
Postoperative best-corrected visual acuity (BCVA) progression in logarithm of the minimum angle of resolution (logMAR). *Significant difference compared to the preoperative value (Wilcoxon signed rank test, p < 0.05).
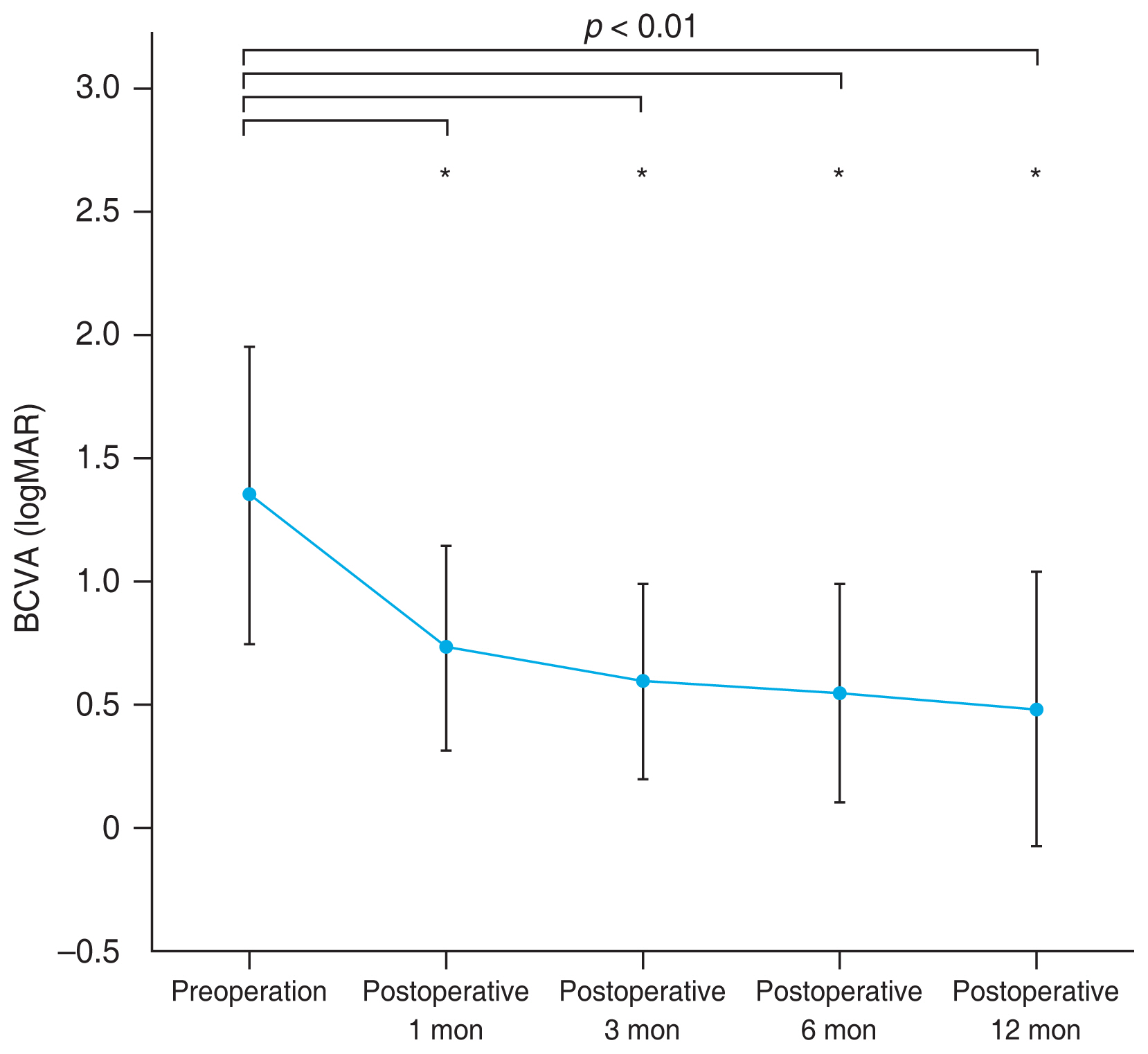
Fig. 2
Postoperative mean absolute error (MAE) and mean error (ME) for Barrett Universal II intraocular lens formula. *Significant difference compared to the preoperative value (repeated measures analysis of variance, p < 0.05).
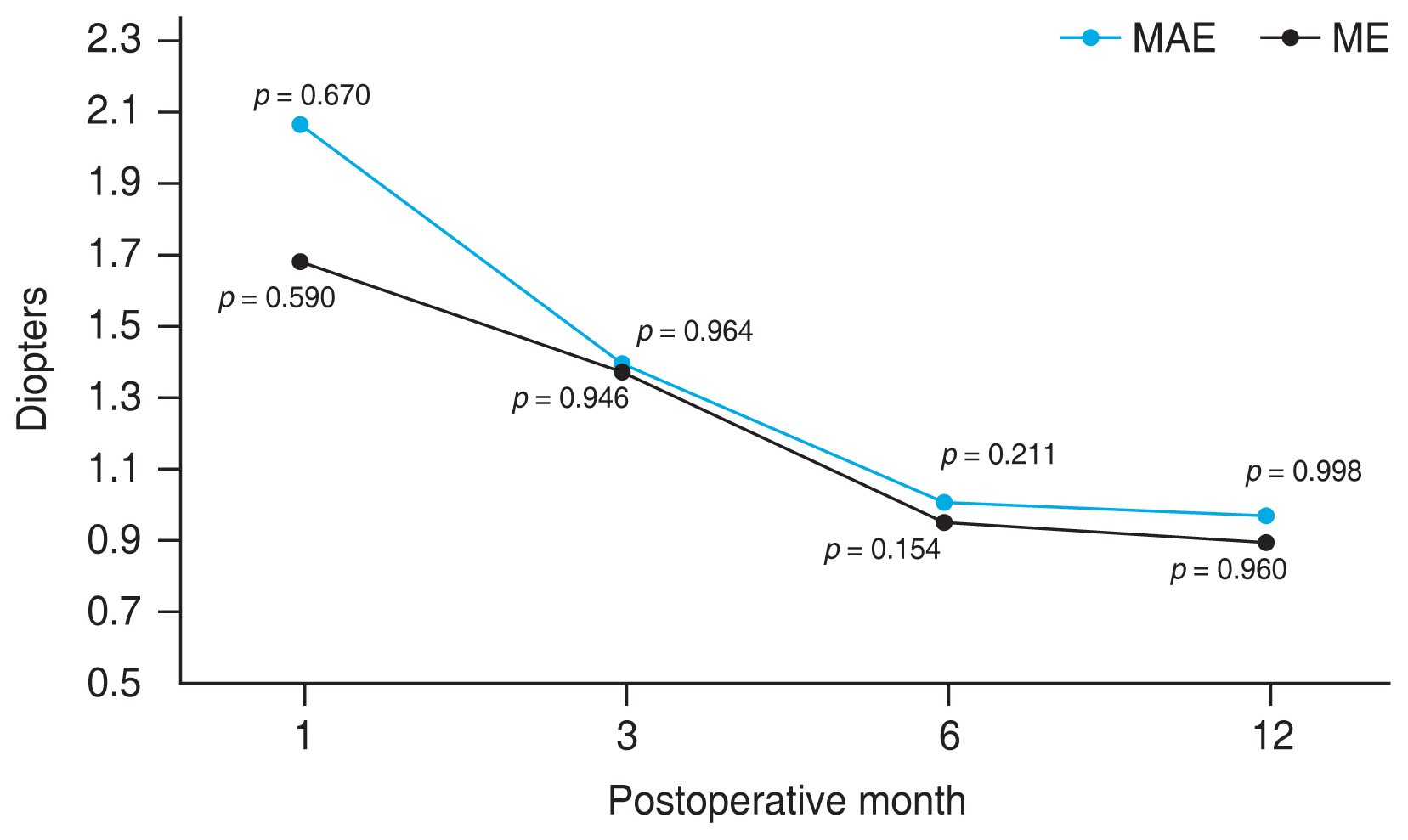
Table 1
Demographics of study population (n = 36)
Table 2
Preoperative ocular biometric factors
Table 3
Donor graft properties
Table 4
Postoperative ocular biometric factors
| Factor | 1 mon (n = 31) | p-value | 3 mon (n = 30) | p-value | 6 mon (n = 28) | p-value | 12 mon (n = 29) | p-value |
|---|---|---|---|---|---|---|---|---|
| BCVA (logMAR) | 0.7 ± 0.4 (0 to 1.9) | <0.001* | 0.6 ± 0.4 (0.1 to 1.7) | <0.001* | 0.5 ± 0.4 (0 to 1.9) | <0.001* | 0.4 ± 0.5 (0 to 2.5) | <0.001* |
| Endothelial cell density (cells/mm2) | 1,883.73 ± 540.07 (850 to 2,717) | 0.018* | 1,839.00 ± 659.81 (812 to 2,857) | 0.080 | 1,748.16 ± 652.72 (664 to 2,882) | 0.327 | 1,841.92 ± 731.24 (553 to 3,205) | 0.128 |
| Coefficient of variation | 33.92 ± 7.87 (13 to 49) | 0.207 | 33.41 ± 8.31 (16 to 50) | 0.065 | 31.27 ± 8.41 (0 to 45) | 0.212 | 34.72 ± 6.46 (19 to 47) | 0.553 |
| Hexagonal cell shape over time | 47.92 ± 13.65 (31 to 83) | 0.892 | 48.86 ± 9.56 (33 to 66) | 0.031 | 49.27 ± 15.73 (25 to 100) | 0.989 | 47.64 ± 14.15 (21 to 66) | 0.761 |
| Central corneal thickness (μm) | 608.69 ± 104.03 (499 to 779) | 0.059 | 613.25 ± 93.05 (474 to 882) | 0.004* | 584.75 ± 48.57 (487 to 672) | 0.011* | 597.41 ± 86.26 (430 to 842) | 0.001* |
| Keratometry (D) | ||||||||
| K1 | 41.57 ± 1.93 (35.50 to 45.25) | <0.001* | 42.13 ± 1.80 (38.50 to 45.00) | 0.105 | 42.18 ± 1.86 (38.50 to 45.50) | 0.165 | 42.73 ± 1.31 (40.50 to 45.75) | 0.045* |
| K2 | 44.38 ± 1.80 (41.75 to 49.25) | 0.668 | 44.05 ± 1.53 (41.75 to 47.25) | 0.408 | 44.31 ± 1.77 (41.75 to 50.25) | 0.033* | 44.64 ± 1.91 (42.00 to 51.50) | 0.445 |
| Kavg | 42.97 ± 1.56 (39.13 to 46.0) | 0.011* | 43.14 ± 1.47 (40.63 to 46.13) | 0.177 | 43.24 ± 1.57 (41.00 to 47.50) | 0.423 | 43.68 ± 1.38 (41.50 to 47.25) | 0.276 |
| Kast | −2.68 ± 2.07 (−7.50 to −0.25) | 0.015* | −1.87 ± 1.57 (−5.50 to 0) | 0.891 | −0.47 ± 1.73 (−6.25 to −0.25) | 0.580 | −1.93 ± 1.79 (−7.50 to −0.25) | 0.984 |
| Spherical equivalent (D) | 0.29 ± 3.01 (−6.13 to +8.00) | 0.135 | 0.05 ± 1.54 (−2.00 to +6.00) | 0.530 | −0.47 ± 1.06 (−2.88 to +1.88) | 0.381 | −0.57 ± 1.06 (−2.50 to +1.50) | 0.299 |
Table 5
Postoperative refractive outcomes



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print




