Efficacy and Rapidity of Potassium Hydroxide Mount and Modified Chicago Sky Blue 6B Stain with Potassium Hydroxide in Fungal Keratitis Detection
Article information
Abstract
Purpose
To compare the efficacy and rapidity of direct microscopic detection of fungal elements from corneal ulcers between 10% potassium hydroxide (KOH) and 1% Chicago Sky Blue 6B (CSB) in 10% KOH (CSB-KOH).
Methods
Thirty patients with clinically suspected fungal keratitis were recruited. Participants with impending corneal perforation were excluded. Two slides were smeared with corneal ulcer scrapings from the ulcer’s edge and base for comparison of fungal staining solutions. One slide was infused with KOH, and the other slide was filled with CSB-KOH. Additional scraping was collected for inoculation on Sabouraud dextrose agar for fungal culture. The sensitivity, specificity and rapidity of both stainings were analyzed.
Results
The sensitivity of fungal culture, KOH, and CSB-KOH were 43.75% (95% confidence interval [CI], 19.75%–70.12%), 62.50% (95% CI, 35.43%–84.80%), and 87.50% (95% CI, 61.65%–98.45%), respectively. The specificity were 100% (95% CI, 69.15%–100%) of both stainings and fungal culture which analyzed from 16 fungal keratitis cases by laboratory and clinical diagnosis. Mean CSB-KOH examination time was quicker than KOH with the mean time difference of 5.6 minutes (95% CI, 3.22–7.98 minutes) and p-value < 0.001.
Conclusions
CSB-KOH was more effective and faster than KOH in detecting fungal elements from corneal ulcers. Therefore, CSB-KOH may be beneficial in diagnosing fungal keratitis and preventing blindness. Moreover, to the best of our knowledge, this is the first use of CSB stain in fungal keratitis detection.
Each year, blindness caused by fungal keratitis affects over half a million people, mainly from Asia and Africa [1]. It accounts up to 45% of total keratitis cases [1,2]. Since the clinical diagnostic sensitivity of fungal keratitis is limited, the relevant tests should be conducted whenever possible. The in vivo confocal microscopy of the cornea allows the clinician to make a real-time diagnosis of fungal keratitis [3]. However, the price and limited availability of this technology may dissuade ophthalmologists from advocating its use in low- and middle-income nations. Corneal smears and culture are the current gold standard for detecting fungal keratitis; however, culture takes a longer duration to generate growth. Fungi cultures may require weeks of incubation [4]; therefore, a diagnosis based solely on culture is frequently delayed and microscopy of corneal smears is generally recommended. Direct microscopy of corneal specimens gives immediate imaging of fungi and permits a provisional diagnosis facilitating treatment initiation. There are several recommended staining methods for microscopic evaluation, including gram stain, potassium hydroxide (KOH), calcofluor-white combined with KOH, lactophenol cotton blue, trypan blue and calcofluor-white combined with Giemsa stain [1,2,5–9]. The sensitivity of dyes detecting fungal keratitis has been reported to be 36% to 50% using gram stain [1], 75.7% using trypan blue [7], 85% using lactophenol blue [1], 61% to 94% using KOH [1], 96.6% using calcofluor-white combined with KOH [9], and 98.3% using calcofluor-white combined with Giemsa stain [9].
Although calcofluor-white has the highest sensitivity, it requires an additional cost of a fluorescence microscope, which may not be available in areas with limited resources. Therefore, KOH stain is practical to be conventional standard test [6,10]. However, the presence of artifacts in stained corneal material may lead to false-positive results [1]. KOH requires expertise to correctly interpret sample results [11] with a rate of false negatives up to 15% [5]. Chicago sky blue 6B (CSB) is a contrast dye used to detect common superficial fungal skin infections. CSB stains superficial skin fungi blue against a purple background, hence improving the identification of fungal elements [12,13]. CSB-KOH has a shorter examination time in detecting fungi in skin samples than KOH and calcofluor-white [5]. In addition, the sensitivity of CSB-KOH is comparable to calcofluor-white [5], but superior to KOH [14].
The purpose of the current study was to assess the efficacy and detection time of 1% CSB in 10% KOH staining with 10% KOH mount for the direct microscopic diagnosis of fungal keratitis.
Material and Methods
Ethics statement
This true experimental study was approved by the Institutional Review Board of the Faculty of Medicine, Naresuan University (No. 617/59). Informed consents were obtained from all participants.
Inclusion and exclusion criteria
Thirty patients with clinically suspected fungal keratitis aged 18 years or older who attended the outpatient ophthalmology clinic were included after they were evaluated by corneal specialists for enrollment. However, patients with imminent perforated corneal lesions were excluded due to the increased risk of perforation during sample collection. A thorough antifungal treatment history, including medications and administration route, were obtained.
Sample collection
The area around the eyes was disinfected with a standard sterile solution containing 10% povidone iodine. The affected eyes were prepared with topical 0.5% tetracaine for anesthesia. Corneal ulcers were scraped from the base and edge of the lesion with a sterile, curved-tip number 15 blade. The first two scrapings were obtained and deposited on two uncoated microscope glass slides (Healthcare Distribution Alliance) for fungal staining, and the third scraping was inoculated on Sabouraud dextrose agar for fungal culture and identification.
Chemicals and preparation
Chemicals of 10% KOH (Merck KGaA) and 1% CSB (Sigma Aldrich) in 10% KOH were prepared by weight per volume in deionized water.
Wet slide preparation and examination sequence
Two scrapings from each eye were smeared onto two glass slides. The first wet slide was prepared with 10% KOH without warming, and the second slide was prepared with 1% CSB in 10% KOH without warming. Direct microscopy was performed immediately after applying 10% KOH or 1% CSB in 10% KOH and placing a cover slip on the slide. Low (×10) and high (×40) magnifications were utilized to detect and confirm the presence of fungi. The timer was started after putting the cover slip and stopped when a final result was obtained. In cases where the initial fungal structure was not sufficiently prominent to facilitate a decision, the search extended to the next observable fungal structure. A negative outcome was determined after a thorough scan of the entire area yielded no structures arousing suspicion. One examiner performed all microscopic examinations.
Statistical analysis
Continuous data are presented as means ± standard deviations. Independent t-tests were used to compare continuous data. A p-value less than 0.05 was considered statistically significant. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy with 95% confidence intervals (CIs) were measured. Statistical analyses were performed using a statistic computer program [15].
Results
A total of 30 eyes from 30 patients were enrolled in the study from February 2017 until July 2020. All eyes were clinically suspected of fungal keratitis and diagnosed by corneal specialists at the outpatient ophthalmology clinic. Of the 30 patients, 17 (56.7%) were male and 13 (43.3%) were female. Mean age was 56.8 ± 16.8 years (range, 20–82 years). The subgroup mean age analysis revealed that the group with positive KOH or CSB-KOH staining results had a mean age of 54.57 ± 17.12 years, while the group with negative results had a mean age of 58.75 ± 20.51 years. Six of the 30 patients were treated with antifungal medication (both topical and systemic) prior to enrollment in the study since they were referred from other hospitals. The outcome of the patients in this study is demonstrated in Fig. 1. The detection and comparison findings for fungal keratitis between KOH and CSB-KOH are shown in Tables 1, 2 and Fig. 2A–2D. The mean negative-outcome times for KOH and CSB-KOH staining were 7.45 ± 1.38 and 2.35 ± 0.22 minutes, respectively. The difference in mean negative-outcome time was significant (5.1 minutes; 95% CI, 2.30 to 7.88 minutes; p = 0.001). The mean positive-outcome time was shorter for CSB-KOH than for KOH staining (mean difference, 4.22 minutes; 95% CI, −0.37 to 7.47 minutes; p = 0.071). Four out of 30 cases were positive for fungal detection on CSB-KOH staining, but no fungal detection was observed on KOH staining (mean positive time, 3.43 ± 1.22 minutes). All seven samples that were positive for fungal keratitis on culture were also positive on CSB-KOH staining, but only five of them were positive with KOH staining. Pythium insidiosum was detected in culture from corneal biopsy. Whereas corneal scrapings were negative for both stainings and fungal culture.

The outcome of the enrolled patients. KOH = potassium hydroxide; CSB = Chicago Sky Blue 6B; CSB-KOH = 1% CSB in 10% KOH.

Sensitivity and specificity of fungal culture, 10% KOH, and from CSB-KOH 16 fungal keratitis cases by laboratory and clinical diagnosis
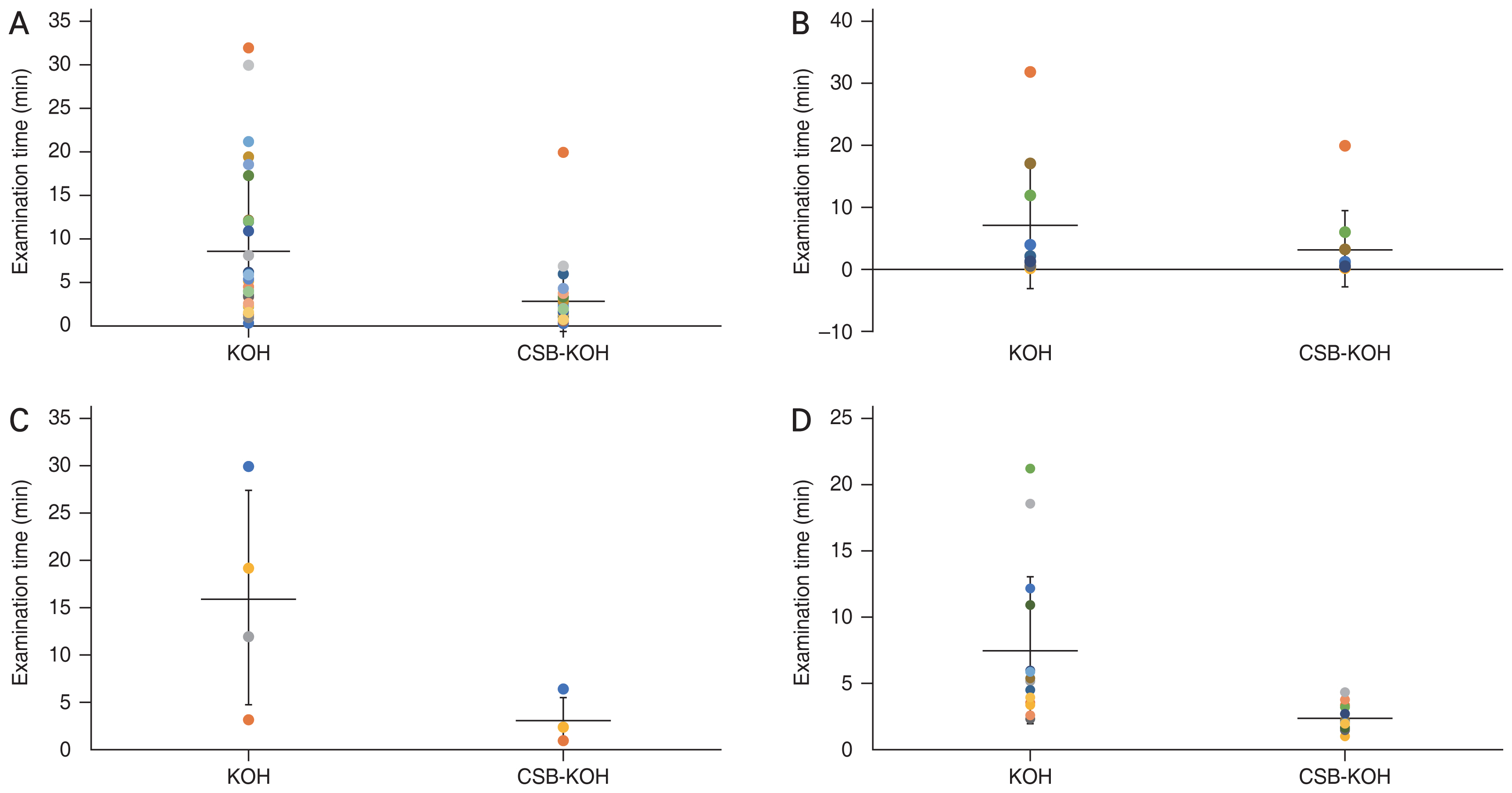
Examination times in 10% potassium hydroxide (KOH) and 1% Chicago Sky Blue 6B (CSB) in 10% KOH (CSB-KOH). CSB-KOH required shorter examination time than 10% KOH mount alone in (A) all 30 specimens, (B) 10 fungus-positive specimens, (C) four 10% KOH false-negative findings, and (D) 16 fungus-negative specimens.
Discussion
This study assessed the efficacy and detection time of CSB-KOH staining with a 10% KOH mount for the microscopic examination of fungal keratitis. We found that CSB-KOH exhibited higher levels of efficacy and a shorter detection time than KOH. Furthermore, the detection time in CSB-KOH was shorter for both fungus-positive and fungus-negative specimens compared to KOH. The better efficacy of CSB-KOH was validated by its high sensitivity, specificity, accuracy, positive predictive value, and negative predictive value. Our sensitivity of KOH in positive fungal culture specimens; 71.43 % (five out of seven) is similar to previously reported of 61% to 94% [1]. Moreover, the false-negative findings of KOH demonstrated the high performance of CSB-KOH.
CSB-KOH showed greater efficacy and a rapid detection time due to its contrast staining property. In our CSB-KOH staining, fungal elements were easily recognizable by the appearance of a contrasting blue stain against a purplish or pink background (Fig. 3A–3D). This contrast stain is comparable to the CSB-KOH detection of superficial fungal skin infections in previous literatures [5,16]. Since the fungal elements were easily identifiable, the time needed to examine CSB-KOH stains was significantly less than that required for KOH tests (Fig. 2). This finding coincided with a previous report on the detection of dermatophytes, wherein the CSB-KOH stain demonstrated the highest detection rate of 94.5% at 5 minutes, while KOH showed only 84.9% at 30 minutes [5]. In comparison, KOH mount relies on clear and transparent fungal structures (Fig. 3), which were difficult to recognize and required more time to assess. Visualizing of KOH mounts for superficial fungal skin infections requires specialized skills [12,16,17] and experience [11] to ensure accurate findings. As a potent alkali, KOH disintegrates and removes cell debris [18] in preparation for microscopic detection of fungal hyphae [10]. We observed that KOH required some time to dissolve cell debris, and the thicker smear necessitated additional time before fungal hyphae could be identified. In CSB-KOH, partial debris digestion was sufficient to stain contrast dye. Similar to our findings, other studies have demonstrated that CSB-KOH staining is highly sensitive [14] and more rapid [5] than KOH staining.
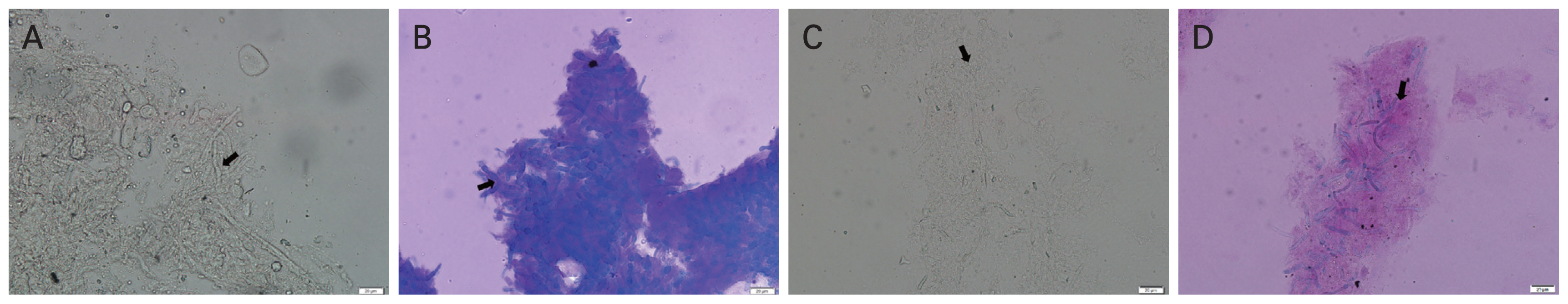
The findings of 10% potassium hydroxide (KOH) and 1% Chicago Sky Blue 6B (CSB) in 10% KOH (CSB-KOH). The final culture results showed (A,B) Aspergillus spp. and (C,D) Fusarium spp. Fungal structures of septate hyphae appear (A,C) as transparent tubular structures among cell debris with occasional dichotomous branching when stained with 10% KOH and (B,D) as bluish hyphae on a purplish to pink background when stained with CSB-KOH (×40 magnification).
Although previous literatures have used a CSB-KOH mixture to detect fungal infections in skin samples, we pioneered the use of CSB-KOH to identify fungal elements in corneal ulcer material to aid in the diagnosis of fungal keratitis. Candida spp. was not detected in our study. Most global fungal keratitis comes from Asia, with the lowest incidence in Europe [1]. The leading etiology of fungal keratitis in Europe is Candida spp. [19,20] but not Asia [21–24]. Immunosuppression is a significant risk factor for reported fungal keratitis in Europe [19,20]. In contrast, the leading risk factor in Asia is trauma [1,21,24]. Our results resemble recent reports from Northern Thailand [23,25] that trauma is the leading risk factor for fungal keratitis. In addition, none of our patients were immunocompromised. It has been reported that Candida spp. take up CSB poorly at 20 minutes but found clearly stained on the following day [12]. We recommend reevaluating negative CSB-KOH after 20 minutes or on the following day if candidiasis is suspected. Therefore, CSB-KOH staining exhibits a minimal advantage over KOH in Candida detection. Several conventional direct examination techniques, including KOH mount, have been reported to detect Pythium [26,27]. However, one of our patients showed negative CSB-KOH and KOH findings, but a subsequent culture from corneal biopsy indicated the presence of Pythium insidiosum. This is likely since the corneal scraping specimen from this patient contained only a few pathogens. Future research may examine contrast staining with CSB-KOH in Pythium insidiosum keratitis as this is a potentially viable topic for inquiry.
The key strengths of the present study are the color contrast property of CSB, which facilitated easy recognition and identification (Fig. 3) and the reduction of examination time (Fig. 2). This may result in an increase in the microscopist’s productivity. The shorter examination time in the CSB-KOH staining method corresponds to previous research indicating a higher detection rate at 5 minutes in CSB-KOH than KOH [5]. In addition, specimens with negative results in CSB-KOH required shorter examination time than in KOH. Despite the occurrence of false-negative KOH readings, the detection of hyphae using CSB-KOH provided more evidence in establishing the efficacy of CSB contrast dye. Four out of 30 false-negative KOH results (13%) in our study matched the reported false-negative KOH rate of up to 15% [28]. Similar to the present findings, Prakash et al. [5] demonstrated that CSB-KOH is superior to KOH in detecting dermatophytes. The CSB-KOH stain is preferable to other staining methods for diagnosing dermatophytosis due to its cost-effectiveness, ease of use, immediate detection, and better comprehension of fungal morphology. Similar to the present study, the key advantage of CSB stain was no additional required equipment.
The present study is subject to some limitation. Some of our patients were previously treated with antifungal medications. The outcomes of six fungal keratitis patients that previously treated with topical antifungal with or without oral antifungal were as follows, two found to be Aspergillus spp. by culture, three detected by direct microscopy, and one not found by all methods. Thus, cases with prior antifungal treatment may affect the outcome of laboratory investigations. Future studies on fungal keratitis should exclude cases with previous antifungal therapy.
To our knowledge, the implementation of CSB-KOH staining in clinical practice for the detection of fungal keratitis has not been extensively studied previously. Experienced examiners, proficient in direct KOH examination, may not perceive a significant advantage in using CSB-KOH staining compared to those less experienced in the field. However, a notable advantage of CSB-KOH staining is the reduced examination time required for slides with negative findings. This efficiency is advantageous for both experienced practitioners and novices, suggesting its potential utility in clinical laboratory settings.
In conclusion, for the direct examination of fungal keratitis, CSB-KOH staining exhibited superior sensitivity, greater efficacy and a shorter detection time than KOH. This method may aid in the diagnosis of fungal keratitis especially in areas with limited resources and help in prevention of blindness in Asia and Africa, where the disease is prevalent.
Acknowledgements
The authors would like to express their gratitude to the Faculty of Medicine, Naresuan University (Phitsanulok, Thailan) for providing financial support; to the highly trained cornea specialists of Naresuan University Hospital; Dr. Rossukon Khotcharrat, Dr. Panotsom Kgowyutagon, and Dr. Taniya Bhoopat for their assistance in patient selection; to our clinical pathologist, Dr. Thanyasiri Jindayok for laboratory information; and finally to Dr. Nattamol Kosaiyaganonth and Dr. Veeraphatra Wongsantimeth for patient information.
Notes
Conflict of Interest: None.
Funding: This study was supported by the Faculty of Medicine, Naresuan University (No. MD2562C005).
