 |
 |
| Korean J Ophthalmol > Volume 31(5); 2017 > Article |
Abstract
Purpose
To evaluate the change of residual volume of eye drop after instillation in patients with 23-gauge microincision vitrectomy surgery (MIVS).
Methods
Patient who were treated 23-gauge MIVS from November 2014 to July 2015 were included. The residual volume was defined as the amount of remnant eye drop in patient's eyes after instillation, calculated as the difference between instillation volume and spilled volume of eye drop. Calculation of residual volume of eye drop was performed one day before surgery, and daily from postoperative day 1 to day 5.
Results
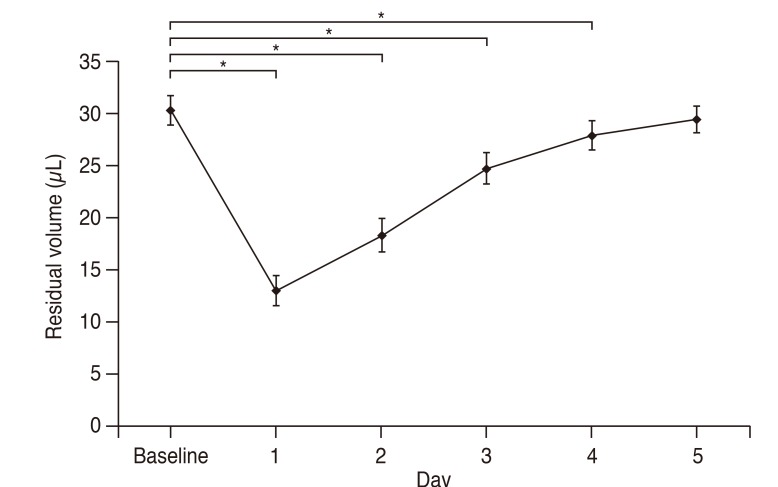
Forty consecutive patients were included. The residual volume of eye drop decreased from 30.3 ± 1.4 µL at baseline to 13.0 ± 1.5 µL at day 1, 18.3 ± 1.6 µL at day 2, 24.7 ± 1.5 µL at day 3, and 27.9 ± 1.4 µL in day 4, postoperatively (p < 0.001, respectively). The volume at postoperative day 5 was 29.4 ± 1.3 µL, but it was not different from the volume at baseline (p = 0.105). The change of residual volume was significantly correlated with postoperative chemosis (r = 0.672, p < 0.001) and effected by the number of quadrant with postoperative chemosis (p < 0.05).
Conclusions
This study shows that postoperative residual volume of eye drop after instillation decreased until postoperative day 4, and postoperative chemosis affects the change of residual volume. Thus, checking proper use of eye drops and teaching about instillation technique by physician is necessary for patients with 23-gauge MIVS.
Topical instillation of eye drops is used to treat most ocular diseases [1] and is recommended after vitreoretinal surgery to control postoperative complications. Postoperative antibiotics are commonly administered to lower surface bacterial contamination and to suppress the growth of infectious pathogens [2]. Anti-inflammatory eye drops, such as topical corticosteroids, are applied to control postoperative intraocular inflammation after vitrectomy, which may exacerbate the underlying inflammatory process [3].
However, 57% of patients have shown problems with the instillation of eye drops [4]. It has previously been reported that there is a considerable degree of imprecision in the dose administered [5], which may be a cause for concern because the pharmacological effect of a compound can be greatly influenced by the volume instilled [6,7,8]. Actually, quite often patients have complaints about the use of topical eye drops after vitreoretinal surgery. There are various factors that result in inadequate eye drop instillation as follows; poor technique, especially in those with poor manual dexterity, poor vision, limited schooling, and old age [9]. A previous report showed that 9.6% of patients who received vitrectomy with retrobulbar anesthesia had moderate to severe postoperative chemosis [10]. Postoperative chemosis also could be one of the factors that lead to inadequate eye drop instillation.
In this study, we investigated the change in residual volume of eye drop instillation after 23-gauge microincision vitrectomy surgery (MIVS), and analyzed factors associated with the changes in residual volume of an eye drop.
This prospective cohort study was performed at the Department of Ophthalmology at Kyungpook National University Hospital. The study protocol was approved by the institutional review board of Kyungpook National University Hospital and conducted in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from each eligible participant.
Subjects were all consecutive patients who received 23-gauge sutureless MIVS at Kyungpook National University Hospital between November 2014 and July 2015 for various vitreoretinal disorders including rhegmatogenous retinal detachment, epiretinal membrane, macular hole, and vitreous hemorrhage due to retinal vein occlusion. Patients were excluded if they met any of the following criteria: a history of previous ocular surgery other than cataract surgery, preoperative chemosis or conjunctivochalasis, eyelid disease including blepharitis, symblepharon, entropion, and ectropion, orbital disease including orbital tumor, thyroid associated ophthalmopathy, and diabetic mellitus including diabetic retinopathy. Patients with a blind or absent fellow eye were also excluded. In addition, patients were excluded from this study if a surgeon performed corneal or conjunctival sutures during surgery that could have caused irritation. Considering severe reflex tearing, patients that had ocular surface disease before surgery and postoperative corneal epithelial defects or superficial punctuate keratitis were also excluded.
All patients underwent retrobulbar anesthesia with 1 mL of 4% lidocaine and 1 mL of 0.5% bupivacaine. The 23-gauge MIVS was conducted by one right-handed surgeon (DHP).
Eye drops were applied to the patients in a consistent manner by one physician (YKK). Patients were instructed to extend their neck and look up. Their lower eyelid was slightly pulled to expose the lower conjunctival fornix where one drop was instilled.
The residual volume was defined as the amount of remnant eye drop in the patients' eyes after instillation calculated as the difference between the instillation volume and spilled volume of the eye drop as follows.
Thus, 100 µL of 0.1% sodium hyaluronate solution (Kynex; Alcon, Fort Worth, TX, USA) was instilled in each patient with a 200 µL micropipette. Because the volume could not be measured directly, we used the difference in cotton ball weight and density. After instillation, the spilled eye drop after instillation was collected with a cotton ball before the patient blinked. If a blink occurred, the measurement was repeated 1 hour later. The difference in the weight of the cotton ball was measured before and after collection of the spilled eye drop. Residual volume was calculated using the calibration weight with the density of the 0.1% sodium hyaluronate solution. The average density for the 0.1% sodium hyaluronate was 0.98 mg/mL which was measured 50 times repeatedly.
Calculation of the residual volume of the eye drop was performed once preoperatively, and daily from postoperative day 1 to day 5. The change in residual volume was defined as the difference in the volume between the baseline and postoperative day 1.
The European Group on Graves' Orbitopathy (EUGOGO) defined the protocol of measuring chemosis with slitlamp for evaluation of patients with Graves' orbitopathy [11]. While patients were told to adopt a primary gaze, a vertical narrow slit-lamp beam (1 mm) was set at 60° midway between the lateral canthus and limbus. According to the EUGOGO chemosis scoring method, the definition of chemosis includes the following criteria: conjunctiva separated from the sclera and prolapsed in front of the grey line, separation of sclera and conjunctiva is over one third of the total height of the palpebral aperture during slit-lamp examination midway between the lateral limbus and lateral canthus. Our method included two modifications. First, chemosis was measured at two points by slit-lamp biomicroscopy, which were midway between the lateral limbus and lateral canthus and midway between the medial limbus and medial canthus. Second, the conjunctiva was divided into four parts (quadrants) corresponding to 3, 6, 9 or 12 o'clock and chemosis location was assessed in those four areas (superotemporal, superonasal, inferotemporal, and inferonasal) (Fig. 1A, 1B). Chemosis score was calculated as the sum of the grade for the four quadrants (range, 0 to 4). Evaluation of chemosis grade was performed on postoperative day 1.
Statistical analyses were performed using SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA). The Wilcoxon signed-rank test was used to compare the mean residual volume between baseline and each postoperative day. Mann-Whitney and Kruskal-Wallis tests were performed to compare the change in the residual volume between baseline and the postoperative days with associated factors. Univariate linear regression analysis was performed to compare the change in the residual volume and clinical factors. Multiple linear regression analysis performed to assess the effect of each variable on residual volume change. For all statistical tests, a p-value <0.05 was considered significant.
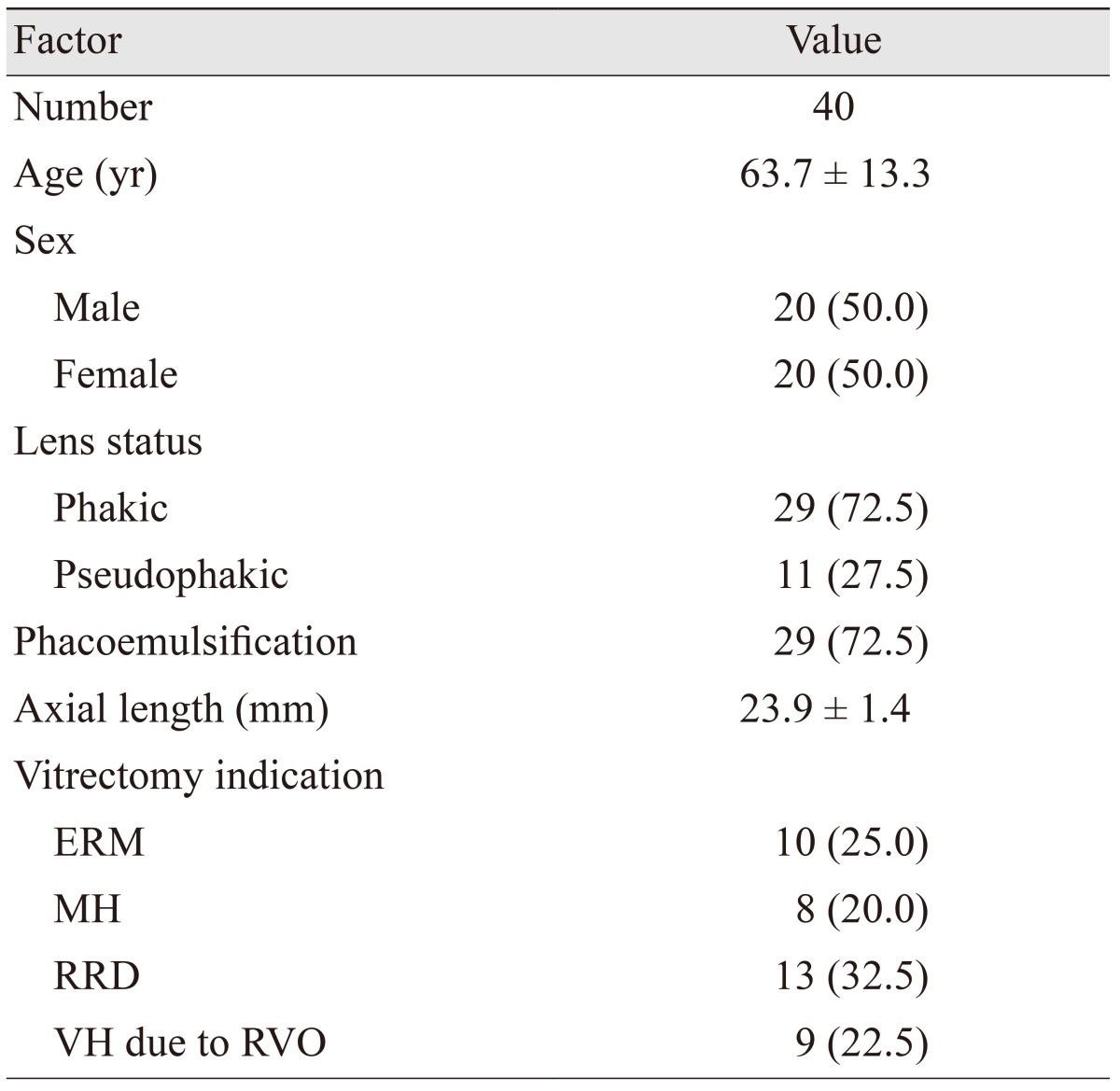
A total of 42 consecutive patients were enrolled in this study. Two patients were excluded because of blepharitis. Forty patients participated in this study; their clinical characteristics are shown in Table 1.
The mean residual volume of the eye drop decreased from 30.3 ± 1.4 µL at baseline to 13.0 ± 1.5 µL on postoperative day 1 (p < 0.001), and the mean residual volume in creased to 18.3 ± 1.6 µL on postoperative day 2, 24.7 ± 1.5 µL on day 3 and 27.9 ± 1.4 µL on day 4 (p < 0.001, respectively). The residual volume on postoperative day 5 was 29.4 ± 1.3 µL; however, it was not different from that at baseline (p = 0.136) (Fig. 2).
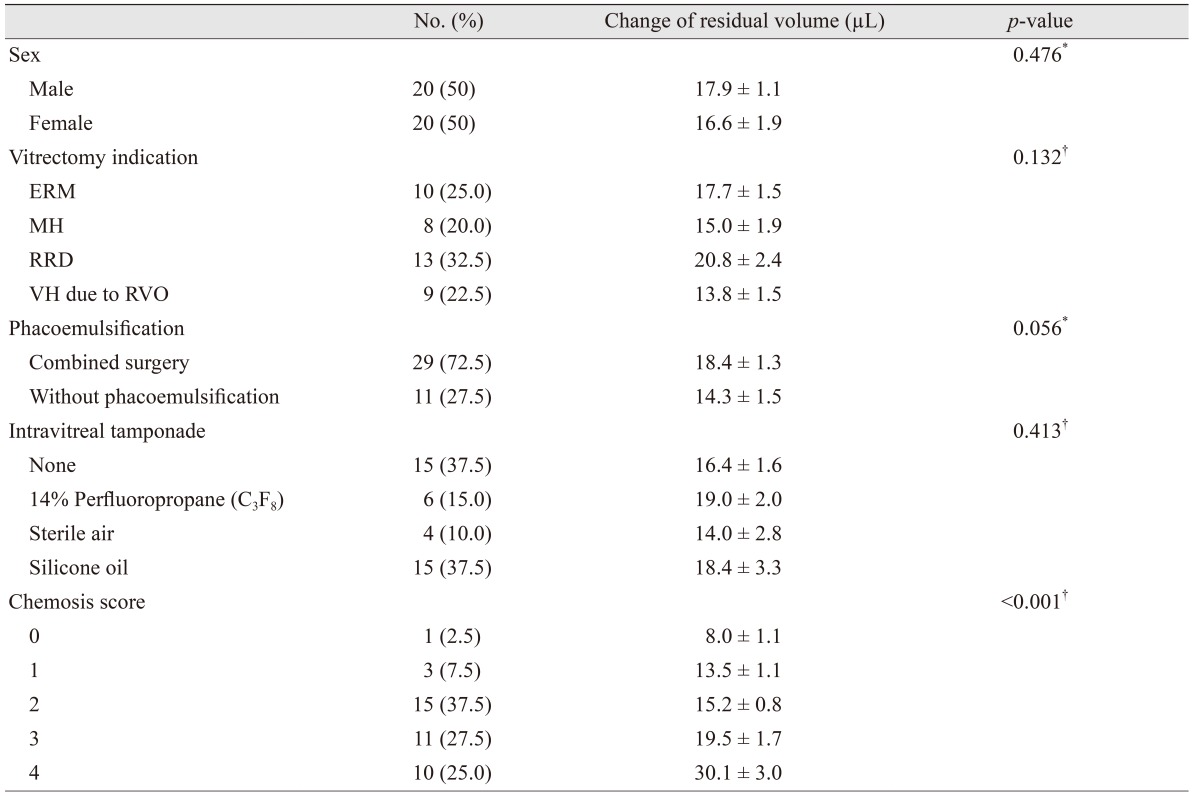
The change in residual volume between baseline and postoperative day 1 was 17.3 ± 1.1 µL (p < 0.001), and it was affected by chemosis score (p < 0.001) (Table 2). Patients with rhegmatogenous retinal detachment tended to have a larger volume change than patients with macular hole, epiretinal membrane and vitreous hemorrhage. However, the indication of vitrectomy did not affect the change in residual volume (p > 0.05). Combined vitrectomy with phacoemulsification did not affect the change in residual volume compared with patients who received vitrectomy without phacoemulsification (p > 0.05). The change in residual volume in cases with 14 % perfluoropropane (C3F8) gas or silicone oil tamponade tended to be larger than in cases with no tamponade, or tamponade with sterile air. However, they were not statistically significant (p > 0.05).
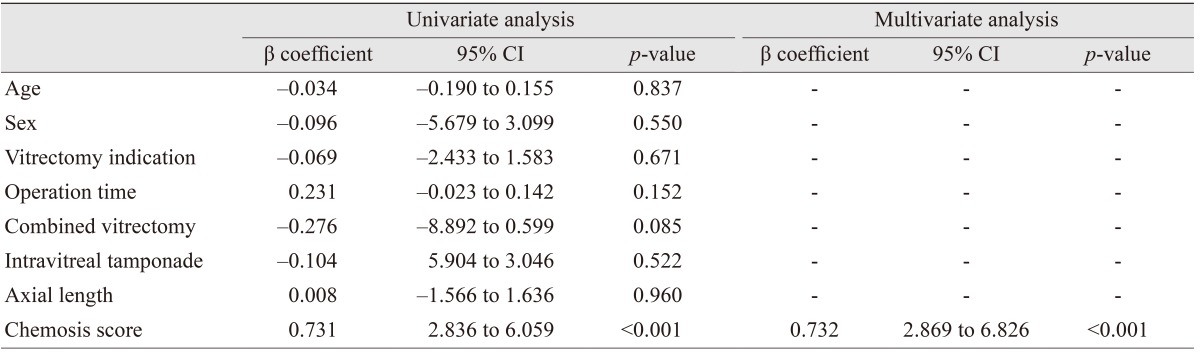
Univariate and multivariate linear regression analyses demonstrated that chemosis score was significantly associated with change in residual volume (p < 0.001) (Table 3). Age, gender, vitrectomy indication, operation time, combined vitrectomy, intravitreal tamponade, and axial length were not associated with residual volume change (p > 0.05, respectively).
Correct instillation of eye drops is essential for their efficacy; therefore, the best method of clinical management for ocular disease is to follow the treatment regimen and to instill eye drops correctly. Ritch et al. [12] reported on the efficacy of accurately instilled eye drops in patients with limited vision; however, many patients had difficulty in following the regimen. Brown et al. [13] found that 83% of glaucoma patients were able to instill eye drops into the eye, and 47% did not touch the eye or the ocular adnexa with the bottle tip. In a more recent, multicenter study with direct observation, Kholdebarin et al. [14] reported that 33.8% of 500 glaucoma patients demonstrated an improper instillation technique; 6.8% missed their eye and 28.8% contaminated the bottle tip.
Various topical ophthalmic agents are used after vitreoretinal surgery [2,3,15]. The maximum volume that the conjunctival sac can hold without overflowing is estimated to be about 30 µL under normal conditions when the patient is upright and not blinking [16]; thus, the mean volume of one eye drop is usually higher than the maximum volume that the conjunctival sac can hold. However, postoperative changes in vitrectomized eyes, such as decreased visual acuity, eyelid swelling and conjunctival chemosis, make it difficult to instill an eye drop properly. Especially, postoperative changes in the conjunctival sac lead to a reduction in remaining eye drop volume after instillation. As a result, the residual volume in the conjunctival sac may be decreased after vitreoretinal surgery.
This study analyzed the postoperative residual volume of the conjunctival sac after eye drop instillation and volume change in the follow-up period after vitrectomy. The decreased residual volume was gradually restored in the postoperative period following vitrectomy, and the residual volume at baseline did not differ from that at postoperative day 5. Thus, until postoperative day 4, the smaller residual volume of an eye drop could affect the efficacy of topical eye drops.
In this study, chemosis score was related to the change in residual volume following vitrectomy. Chemosis, defined as visible swelling of the conjunctiva, may change the anatomical space of the conjunctival sac, which determines residual volume. Our data demonstrated a positive correlation between chemosis score and change in residual volume. As a result, postoperative chemosis reduced the maximum volume of fluid that the conjunctival sac could hold without overflowing.
We evaluated the residual volume by determining the difference between instillation volume and spilled volume because it is impossible to directly evaluate residual volume in the conjunctival sac after eye drop instillation. Accordingly, a few procedures for tear fluid collection have been used previously. Those procedures consisted of absorbing tear fluid with cellulose sponges [17], porous polyester rods [18] or, alternatively, direct aspiration of tear fluid with glass capillary tubes [19]. Those procedures could be effective for residual volume evaluation, but they could cause unavoidable reflex tearing, which can affect measurement results. Thus, this study employed an indirect method and measured eye drop instillation volume and spilled volume. Although this method is not standardized yet and may produce some error, it may be useful for measuring residual volume with minimal reflex tearing.
In conclusion, our results suggest that eye drop instillation postoperative residual volume decreased significantly until postoperative day 4 following 23-gauge MIVS. In addition, postoperative chemosis was a significant factor for the change in residual volume. Therefore, it is necessary for physicians to verify the proper use of eye drops and instillation technique in patients following 23-gauge MIVS, especially in the presence of severe postoperative chemosis.
Acknowledgements
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2014R1A1A2055007, NRF-2017R1D1A1B03027966) and by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (HI16C1501). The sponsor/funding organization played no role in the design or conduct of this research.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
REFERENCES
2. Olson RJ. Reducing the risk of postoperative endophthalmitis. Surv Ophthalmol 2004;49(Suppl 2):S55-S61.


3. Androudi S, Ahmed M, Fiore T, et al. Combined pars plana vitrectomy and phacoemulsification to restore visual acuity in patients with chronic uveitis. J Cataract Refract Surg 2005;31:472-478.


4. Winfield AJ, Jessiman D, Williams A, Esakowitz L. A study of the causes of non-compliance by patients prescribed eyedrops. Br J Ophthalmol 1990;74:477-480.



5. In: Lund W, The pharmaceutical codex: principles and practice of pharmaceutics. 12th ed. London: Pharmaceutical Press; 1994. p. 14.
6. File RR, Patton TF. Topically applied pilocarpine: human pupillary response as a function of drop size. Arch Ophthalmol 1980;98:112-115.


8. Craig EW, Griffiths PG. Effect on mydriasis of modifying the volume of phenylephrine drops. Br J Ophthalmol 1991;75:222-223.



9. Tatham AJ, Sarodia U, Gatrad F, Awan A. Eye drop instillation technique in patients with glaucoma. Eye (Lond) 2013;27:1293-1298.



10. Costen MT, Newsom RS, Wainwright AC, et al. Expanding role of local anaesthesia in vitreoretinal surgery. Eye (Lond) 2005;19:755-761.


11. Barrio-Barrio J, Sabater AL, Bonet-Farriol E, Velazquez-Villoria A, Galofre JC. Graves' ophthalmopathy: VISA versus EUGOGO Classification, Assessment, and Management. J Ophthalmol 2015;2015:249125



12. Ritch R, Jamal KN, Gurses-Ozden R, Liebmann JM. An improved technique of eye drop self-administration for patients with limited vision. Am J Ophthalmol 2003;135:530-533.


13. Brown MM, Brown GC, Spaeth GL. Improper topical self-administration of ocular medication among patients with glaucoma. Can J Ophthalmol 1984;19:2-5.

14. Kholdebarin R, Campbell RJ, Jin YP, Buys YM. Multicenter study of compliance and drop administration in glaucoma. Can J Ophthalmol 2008;43:454-461.


15. Lee SB, Lee DG, Kwag JY, Kim JY. The effect of mydriatics on posterior synechia after combined pars plana vitrectomy, phacoemulsification, and intraocular lens implantation. Retina 2009;29:1150-1154.


16. Mishima S, Gasset A, Klyce SD Jr, Baum JL. Determination of tear volume and tear flow. Invest Ophthalmol 1966;5:264-276.

17. Allansmith MR, Baird RS, Higgenbotham EJ, Abelson MB. Technical aspects of histamine determination in human tears. Am J Ophthalmol 1980;90:719-724.


Fig. 1
(A) The conjunctiva was divided into four parts (quadrants) that correspond to 3, 6, 9 or 12 clock hours. Conjunctival chemosis was judged to be present when the conjunctiva is higher than a third of the total height of the palpebral aperture. (B) An example case of presence of postoperative chemosis in all quadrants of the conjunctiva. SN = superonasal; ST = superotemporal; IN = inferonasal; IT = inferotemporal.
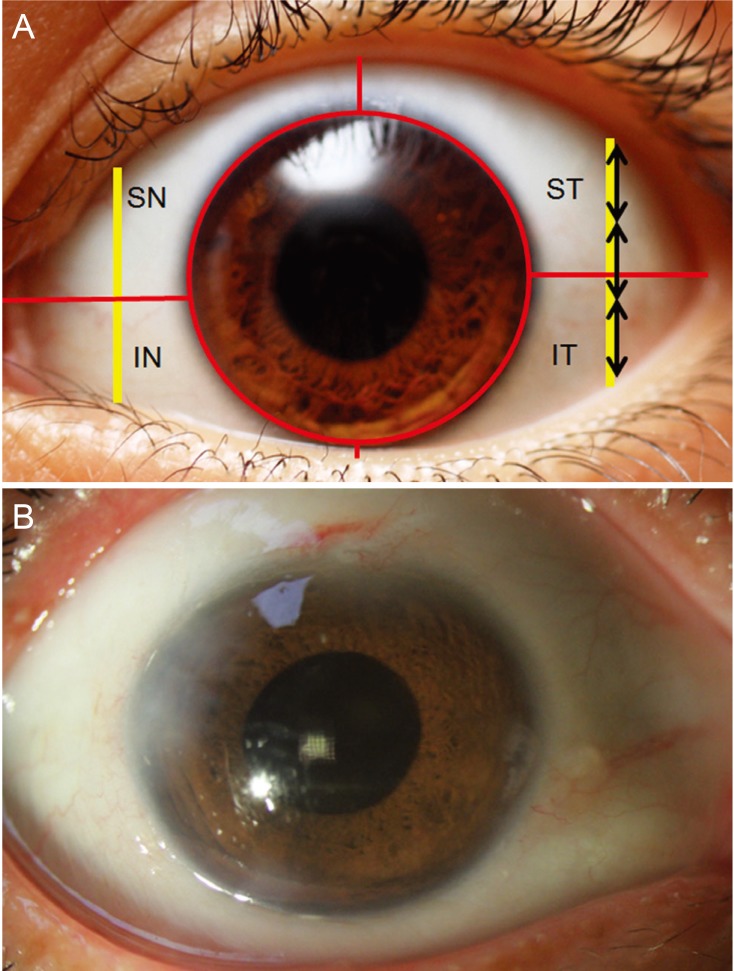
Fig. 2
The residual volume of eye drop changes following 23-gauge microincision vitrectomy surgery according to the postoperative follow-up period. Repeated-measures analysis of variance showed significant decrease in residual volume from the baseline at postoperative day 1, 2, 3 and 4 (p < 0.001, respectively). *p < 0.05 by repeated-measures analysis of variance with Bonferroni correction.
- TOOLS
-
METRICS

-
- 6 Crossref
- 0 Scopus
- 2,622 View
- 8 Download
- Related articles



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



