 |
 |
| Korean J Ophthalmol > Volume 29(6); 2015 > Article |
Abstract
Purpose
To compare the levels of serum cortisol and testosterone in acute and chronic central serous chorio-retinopathy (CSC).
Methods
Serum cortisol and testosterone levels in 30 patients with either acute or chronic CSC were evaluated using chemiluminescent immunoassay.
Results
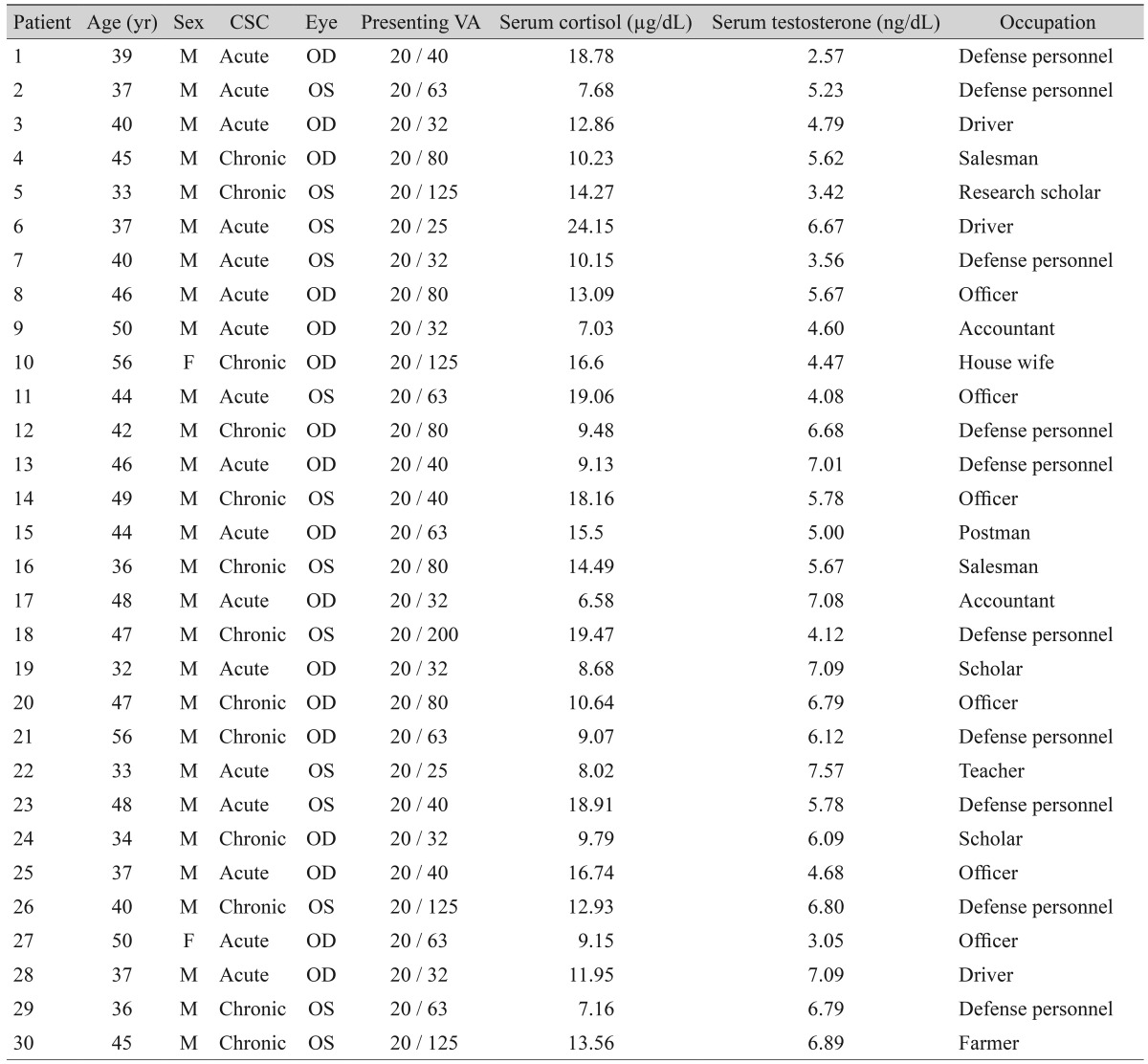
The mean age was 42.43 ┬▒ 6.37 years (range, 32 to 56 years). The mean 8:00 to 9.00 a.m. serum cortisol level was 12.61 ┬▒ 4.74 ┬Ąg/dL (range, 6.58 to 27.42 ┬Ąg/dL). The mean serum testosterone level was 5.88 ┬▒ 1.57 ng/dL (range, 2.81 to 9.94 ng/dL). The mean visual acuity was 20 / 65.07 ┬▒ 40.56 (range, 20 / 25 to 20 / 200). There was no statistically significant difference in the mean levels of serum cortisol and testosterone between the acute and chronic cases (p > 0.05), but there was a statistically significant difference in the mean presenting visual acuity in the two groups (p < 0.05).
Conclusions
All except one patient in the acute group had normal levels of serum cortisol. Testosterone levels were within the normal range in both the acute and chronic cases of CSC. There is unlikely to be any statistically significant difference in the mean levels of serum cortisol and testosterone between the acute and chronic cases, but there may be a statistically significant difference in the mean presenting visual acuity in these groups.
Central serous chorioretinopathy (CSC) is characterized by an idiopathic circumscribed serous retinal detachment of the central macula caused by the leakage of fluid through the retinal pigment epithelium (RPE) [1]. Presenting symptoms are minor blurring of vision, central scotoma, metamorphopsia or micropsia, increasing hypermetropia, and dyschromatopsia [1,2]. There may also be a loss of contrast sensitivity [1,2].
The pathogenesis of CSC is still not clearly understood. Numerous hypotheses have been put forward. A combination of choroidal hyperpermeability and impaired RPE function leads to pooling of fluid in the sub-RPE space with eventual leakage through the RPE into the subretinal space [2].
As evidenced by previous reports or studies, a relationship appears to exist between glucocorticoids and CSC [3,4,5,6]. Garg et al. [3] found significantly higher serum cortisol levels in acute CSC cases as compared to age-matched controls. Pastor-Idoate et al. [4] reported high levels of serum cortisol in a case of adrenocortical adenoma. Zakir et al. [5] also identified elevated levels of serum cortisol but normal levels of testosterone in acute CSC cases. In contrast, Thoelen et al. [6] speculated that in the pathogenesis of CSC, the deregulations of sympathetic activity play a major role and elevated glucocorticosteroid levels are involved only indirectly. Chalisgaonkar et al. [7] found no precise correlation between serum cortisol levels and acute cases of CSC. Similarly, Tufan et al. [8] reported normal levels of serum cortisol and testosterone in chronic CSC. Therefore, the association between acute or chronic CSC and serum levels of cortisol appears to be inconsistent.
There also appears to be a relationship between plasma testosterone and CSC. Just as CSC has a tendency to affect more males than females and to decrease with age, plasma testosterone, which is a male hormone, also declines with age. Therefore, it is likely that testosterone may play a role in predisposing males to CSC. Moreover, androgen receptors have been found in human RPE cells [9]. Ahad et al. [10] was the first to report a case of CSC that occurred in a patient on long-term systemic testosterone therapy for hypogonadotropic hypogonadism. Similarly, Grieshaber et al. [11] reported a 45-year-old, non-pregnant female patient who developed CSC while receiving testosterone treatment for general loss of energy who recovered fully upon discontinuation of the medication. These studies show that there may be a direct association between the serum testosterone level and CSC. However, studies by Zakir et al. [5] and Tufan et al. [8] found levels of serum testosterone to be normal in cases of CSC. Therefore, the exact association of serum testosterone with CSC is still not clear.
These previous studies have either compared the differences in the levels of serum cortisol and testosterone between cases of CSC and normal controls or measured the levels of these hormones without making a comparison. However, if there is a difference in the mean levels of serum cortisol and testosterone between acute and chronic CSC patients, it might shed some further light on the etiopathogenesis of CSC and the reason why some patients have chronic or recurrent CSC whereas other patients do not experience a recurrence after an episode of acute CSC. This information might in turn aid in the modifications of the treatment of CSC. To the best of our knowledge, no study has been conducted to identify the differences in the mean levels of serum cortisol and testosterone in acute and chronic cases of CSC. Therefore, we undertook the present study to document our experience of the possible difference in the levels of serum cortisol and testosterone in acute and chronic cases of CSC.
The study was conducted at the department of ophthalmology of a medical college in North-East India between September 2012 and June 2014. Approval from the institute's ethical committee was obtained, and the study was carried out in accordance with the ethical standards of the 1964 Declaration of Helsinki. The inclusion criteria were as follows: (1) patients with unilateral CSC, either acute or chronic; and (2) patients who were willing to provide written informed consent. Exclusion criteria were as follows: (1) patients with both acute and chronic CSC together in one or both eyes; (2) patients who had a history of using corticosteroids, steroid hormones in any form-systemic steroids (like oral/intravenous/intramuscular), topical eye drops, skin creams, intranasal/inhalational sprays, etc., in the past one month; (3) any recent surgery or trauma; (4) alcohol abuse or dependence; (5) major depression; and (6) other major eye or systemic diseases.
All the patients underwent complete ophthalmic examination. Their visual acuity was tested using illuminated Snellen charts. The refractive status was determined with both automated and retinoscopic methods, using appropriate cycloplegia when needed. Detailed slit-lamp biomicroscopy and indirect ophthalmoscopy were conducted after the pupils had been dilated. Fundus fluorescein angiography (FFA) was carried out using a fundus camera Visucam lite (Carl Zeiss Meditec AG, Jena, Germany) and optical coherence tomography (OCT) with spectral domain OCT (Model 500; Cirrus HD-OCT, Carl Zeiss Meditec, Dublin, CA, USA). The diagnosis of CSC was confirmed by two independent ophthalmologists. The cases were classified as acute or chronic depending on the clinical, FFA and OCT findings.
An acute case of CSC was defined as the first episode of CSC that had symptom duration of less than three months with a well-circumscribed round or oval area of serous elevation of the neurosensory retina at the macular region. These acute cases also showed dye leakage either as a single leak or multiple leaks on FFA, with the leakage pattern being either the inkblot type or the smokestack type, as well as the presence of serous neurosensory retinal detachments either with or without RPE detachments on OCT.
Chronic CSC was defined as CSC that had persisted for more than three months and showed clinical elevation of the neurosensory retina with fine RPE changes, like mottling and atrophy with pigmentation, revealing window defects with small leaks and mottled atrophic changes of RPE of a non-specific pattern on FFA and the presence of serous neurosensory retinal detachments with or without RPE detachments on OCT.
Estimations of the 8:00 to 9:00 a.m. serum cortisol and testosterone levels were carried out in all the patients using a chemiluminescent immunoassay method (Beckman Coulter, Fullerton, CA, USA; supplied by Beckman Coulter India Pvt. Ltd., Mumbai, India); 5 mL blood were collected in a plain vial and allowed to clot for 15 minutes at room temperature. The serum was separated by centrifuging the sample at the rate of 2,500 rpm for ten minutes. The serum was used for the analysis of cortisol and total testosterone levels in an automated Access 2 immunoassay system (Beckman Coulter) as per the manufacturer's protocol. Proper standardization and internal quality control were ascertained as per these guidelines before the assays were conducted. The normal reference levels of serum cortisol and testosterone at our laboratory were determined as 6.70 to 22.60 ┬Ąg/dL and 1.75 to 7.81 ng/dL, respectively.
Descriptive statistics were used to calculate the mean ┬▒ standard deviation and percentages. An independent sample t-test was employed to identify any difference between the two groups. A partial correlation test was used to determine the difference in the levels of serum cortisol and testosterone by controlling for the effect of age and gender. A 5% level of significance was adopted. Therefore, a p-value less than 0.05 was considered statistically significant. Statistical analysis was performed using the IBM SPSS ver. 22.0 (IBM Co., Armonk, NY, USA).
A summary of the characteristics of the patients along with their serum cortisol and testosterone levels is given in Table 1. There were 17 acute and 13 chronic CSC cases. The mean age was 42.43 ┬▒ 6.37 years (range, 32 to 56 years). The mean 8:00 to 9:00 a.m. serum cortisol level was 12.61 ┬▒ 4.74 ┬Ąg/dL (range, 6.58 to 27.42 ┬Ąg/dL). The mean serum testosterone level was 5.88 ┬▒ 1.57 ng/dL (range, 2.81 to 9.94 ng/dL). The mean visual acuity was 20 / 65.07 ┬▒ 40.56 (range, 20 / 25 to 20 / 200). There was no statistically significant difference in the mean levels of serum cortisol and testosterone between the acute and chronic cases (p > 0.05), but there was a statistically significant difference in the mean presenting visual acuity in the two groups (p < 0.05).
No statistically significant difference in the levels of these hormones could be found by controlling for the effect of age (p = 0.937 for serum cortisol and p = 0.545 for testosterone) and gender (p = 0.963 for serum cortisol and p = 0.491 for testosterone) using a partial correlation test.
Clinically, acute cases revealed a well-circumscribed round or oval area of serous elevation of neurosensory retina at the macular region. Chronic cases showed a mild elevation of the neurosensory retina with fine RPE changes, like mottling and atrophy with pigmentation.
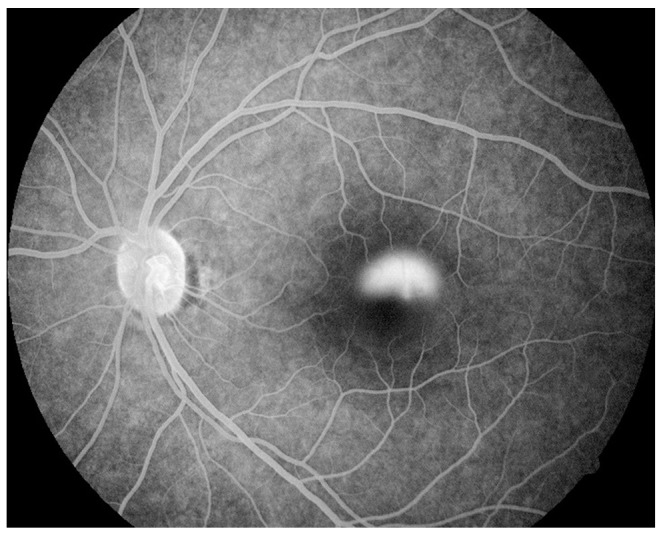
FFA revealed leakage of dye either as a single leak or multiple leaks in acute CSC. The leakage pattern was either the inkblot type or the smokestack type (Fig. 1) in these cases. Two eyes had a smokestack pattern, and the rest had inkblot type leaks. Chronic cases showed window defects with small leaks and mottled atrophic changes of the RPE with a nonspecific pattern (Fig. 2). OCT confirmed the presence of serous neurosensory retinal detachments with or without RPE detachments.
CSC is an idiopathic disease characterized by serous detachment of the neurosensory retina in the macular region secondary to a focal retinal pigment epithelial defect. Risk factors are type A personality [12], corticosteroid use in various forms [13,14,15], age [16], male gender [17], increased endogenous cortisol production [3], systemic lupus erythematosus [18,19], pregnancy [20,21,22], antibiotic use, alcohol use, allergic respiratory disease [22], untreated hypertension [22,23], psychopharmacologic medication [23], organ transplantation [24], hemodialisis [25], and cortisol-producing tumors [4,5]. It is usually self-limited with spontaneous resolution at four to six months, but a few cases may progress to chronic disease with prolonged serous macular detachment [26,27].
CSC is said to predominantly affect young and middle-aged males between 20 and 50 years, and its incidence declines with advancing age. However, there are reports of CSC occurring in patients 60 years or older [1,16]. In our study, the age of patients ranged from 32 to 56 years (mean, 42.43 ┬▒ 6.37 years).
The treatment of CSC is not established uniformly. In a retrospective follow-up study, Gilbert et al. [28] found no significant difference between treated and untreated eyes.
Even centuries after Von Graefe described recurrent serous detachment of the macula as "recurrent central retinitis" in 1866, the etiology of this condition continues to elude clinicians despite active research. Some earlier studies have implicated serum cortisol along with CSC. Garg et al. [3] determined the endogenous cortisol levels in urine and plasma samples in 30 patients with acute CSC and compared them with 30 age and sex matched controls. They found that the mean plasma and urine cortisol levels were significantly higher in patients with acute CSC compared to age-matched controls. Similarly, Kapetanios et al. [29] evaluated the levels of endogenous cortisol in 16 patients with acute CSC and compared them with the age and sex matched control group. They also found a significant difference. Zakir et al. [5] reported that CSC is associated with elevated 8:00 a.m. serum cortisol levels. However, Chalisgaonkar et al. [7] did not find a precise correlation of the serum cortisol with CSC. Similarly, Tufan et al. [8] could not identify any correlation of the serum cortisol with chronic CSC. From these studies, it can be inferred that there is an inconsistent relationship between the levels of serum cortisol and CSC. In our study, 30 patients with either acute or chronic CSC were evaluated. There were 17 cases with acute CSC (56.7%) and 13 cases with chronic CSC (43.3%). All except case no. 6 (96.67%) had serum cortisol levels within the normal range.
Studies have been conducted to find the association of testosterone in CSC. Tittl et al. [23] identified corticosteroid use as a risk factor associated with CSC that was distinct from psychopharmacologic medication use and hypertension in their retrospective study of 230 consecutive patients with CSC. Similarly, in an observational case series of 24 patients, Haimovici et al. [30] reported that the serum testosterone levels were within the normal range in 23 of their 24 patients. Zakir et al. [5] were the first to estimate the serum testosterone levels in patients suffering from CSC and compare them with age and sex matched controls. The mean serum levels of testosterone were observed to be within the normal range in both CSC patients and controls. However, they found 32% of CSC patients and 18% of controls to have lower than normal levels of testosterone. In their study, Tufan et al. [8] reported serum testosterone levels within the normal range in patients with chronic CSC. They concluded that the association between these hormones and chronic CSC might be weak. In our study, all cases (100%) had serum testosterone levels in the normal range.
We carried out the present study because no study thus far has revealed a difference in the levels of serum cortisol and testosterone in acute and chronic CSC. If a difference is found, it might shed some further light on the etiopathogenesis of CSC and explain why some patients with CSC have recurrences whereas others do not. However, we did not find any statistically significant difference in the levels of serum cortisol and testosterone in acute and chronic CSC, even after controlling for the effects of age and gender.
In conclusion, it may be inferred from our study that there is unlikely to be any statistically significant difference in the mean levels of serum cortisol and testosterone between the acute and chronic cases of CSC, but there may be a statistically significant difference in the mean presenting visual acuity in the two groups.
Acknowledgements
We would like to acknowledge the help of the Department of Biochemistry, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, in estimating the levels of serum cortisol and testosterone for this study.
Conflicts of interest
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
REFERENCES
1. Meyerle CB, Spaide RF. Central serous chorioretinopathy. In: Albert DM, Miller JW, Azar DT, Blodi BA, Albert & Jakobiec's principles and practice of ophthalmology. 3rd ed. Philadelphia: Saunders Elsevier; 2008. p. 1871-1880.
2. Klais CM, Ober MD, Ciardella AP. . Central serous chorioretinopathy. In: Klais CM, Ober MD, Ciardella AP, Retina. 4th ed. Philadalphia: Elsevier Mosby; 2006. p. 1135-1161.
3. Garg SP, Dada T, Talwar D, Biswas NR. Endogenous cortisol profile in patients with central serous chorioretinopathy. Br J Ophthalmol 1997;81:962-964.



4. Pastor-Idoate S, Pena D, Herreras JM. Adrenocortical adenoma and central serous chorioretinopathy: a rare association? Case Rep Ophthalmol 2011;2:327-332.



5. Zakir SM, Shukla M, Simi ZU, et al. Serum cortisol and testosterone levels in idiopathic central serous chorioretinopathy. Indian J Ophthalmol 2009;57:419-422.



6. Thoelen AM, Bernasconi PP, Schmid C, Messmer EP. Central serous chorioretinopathy associated with a carcinoma of the adrenal cortex. Retina 2000;20:98-99.


7. Chalisgaonkar C, Chouhan S, Lakhtakia S, et al. Central serous chorioretinopathy and endogenous cortisol: is there an association? Indian J Ophthalmol 2010;58:449-450.



8. Tufan HA, Gencer B, Comez AT. Serum cortisol and testosterone levels in chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 2013;251:677-680.


9. Rocha EM, Wickham LA, da Silveira LA, et al. Identification of androgen receptor protein and 5alpha-reductase mRNA in human ocular tissues. Br J Ophthalmol 2000;84:76-84.



10. Ahad MA, Chua CN, Evans NM. Central serous chorioretinopathy associated with testosterone therapy. Eye (Lond) 2006;20:503-505.


11. Grieshaber MC, Staub JJ, Flammer J. The potential role of testosterone in central serous chorioretinopathy. Br J Ophthalmol 2007;91:118-119.



12. Yannuzzi LA. Type A behavior and central serous chorioretinopathy. Trans Am Ophthalmol Soc 1986;84:799-845.



13. Bouzas EA, Karadimas P, Pournaras CJ. Central serous chorioretinopathy and glucocorticoids. Surv Ophthalmol 2002;47:431-448.


14. Carvalho-Recchia CA, Yannuzzi LA, Negrao S, et al. Corticosteroids and central serous chorioretinopathy. Ophthalmology 2002;109:1834-1837.


15. Iida T, Spaide RF, Negrao SG, et al. Central serous chorioretinopathy after epidural corticosteroid injection. Am J Ophthalmol 2001;132:423-425.


16. Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology 1996;103:2070-2079.


17. Spahn C, Wiek J, Burger T, Hansen L. Psychosomatic aspects in patients with central serous chorioretinopathy. Br J Ophthalmol 2003;87:704-708.



18. Cunningham ET Jr, Alfred PR, Irvine AR. Central serous chorioretinopathy in patients with systemic lupus erythematosus. Ophthalmology 1996;103:2081-2090.


19. Carpenter MT, O'Boyle JE, Enzenauer RW, et al. Choroiditis in systemic lupus erythematosus. Am J Ophthalmol 1994;117:535-536.


20. Chumbley LC, Frank RN. Central serous retinopathy and pregnancy. Am J Ophthalmol 1974;77:158-160.


21. Said-Ahmed K, Moustafa G, Fawzy M. Incidence and natural course of symptomatic central serous chorioretinopathy in pregnant women in a maternity hospital in Kuwait. Middle East Afr J Ophthalmol 2012;19:273-276.



22. Haimovici R, Koh S, Gagnon DR, et al. Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology 2004;111:244-249.


23. Tittl MK, Spaide RF, Wong D, et al. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol 1999;128:63-68.


24. Lee CS, Kang EC, Lee KS, et al. Central serous chorioretinopathy after renal transplantation. Retina 2011;31:1896-1903.


25. Basu S, Das T, Padhi TR. Serous retinal detachment and multiple retinal pigment epithelial detachments, following hemodialysis for multi-organ failure. Indian J Ophthalmol 2010;58:261-262.



26. Hussain D, Gass JD. Idiopathic central serous chorioretinopathy. Indian J Ophthalmol 1998;46:131-137.

27. Liu DT, Fok AT, Lam DS. An update on the diagnosis and management of central serous chorioretinopathy. Asia Pac J Ophthalmol (Phila) 2012;1:296-302.


28. Gilbert CM, Owens SL, Smith PD, Fine SL. Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol 1984;68:815-820.



Fig.┬Ā1
Fundus fluorescein angiography image of an acute case of central serous chorioretinopathy revealing leakage of dye, with the leakage pattern being of the smokestack type.




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




