 |
 |
| Korean J Ophthalmol > Volume 27(3); 2013 > Article |
Abstract
Purpose
Antimicrobial peptides have an important role in self-protection of the ocular surface. Human cationic antimicrobial protein (hCAP)-18 is a linear, ╬▒-helical peptide that consists of a conserved pro-sequence called a cathelin-like domain and a C-terminal peptide named LL-37. We investigated the in vitro anti-adenoviral activity of hCAP-18/LL-37 in several adenovirus types, inducing keratoconjunctivitis.
Methods
A549 cells were used for viral cell culture, and human adenovirus (HAdV) types 3 (HAdV3, species B), 4 (species E), 8, 19a, and 37 (species D) were used. The cytotoxicity of LL-37 was evaluated by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay to obtain 50% cytotoxic concentration. After pretreatment of A549 cells with serial dilutions of LL-37 for 24 hours, adenovirus was cultured for seven days, and adenoviral DNA was quantitatively measured by real-time polymerase chain reaction (PCR).
The human adenovirus (HAdV) family consists of 57 known types, which fall into seven species, A to G [1-3]. The most common external ocular viral infections are caused by several HAdV types. In particular, adenoviral conjunctivitis is known to be the major cause of acute infections associated with community and nosocomial epidemics. A recent study revealed that novel types, such as HAdV type 54, are the major types responsible for inducing adenoviral conjunctivitis in Japan [4]. However, no specific anti-adenoviral agent has been established for the treatment of adenoviral infection. There is a need for new antiviral therapeutics with potent activity against HAdV and a favorable therapeutic index.
In innate immunity, antimicrobial peptides have an important role in the self-protection of the body surface. Their spectrum of activity includes Gram-positive and Gram-negative bacteria, as well as fungi and certain viruses [5,6]. They exist in insects, plants, and mammals and have an important role in innate immunity. Cathelicidins are peptide antibiotics that are receiving increased attention. These peptides contain a highly conserved signal sequence and pro-region (cathelin) but show substantial heterogeneity in the C-terminal domain that encodes the mature peptide, which can range in size from 12 to 80 amino acids or more [7]. Human cationic antimicrobial protein (hCAP)-18 is a linear, ╬▒-helical peptide that consists of a conserved prosequence called the cathelin-like domain. The C-terminal 37-amino-acid mature peptide encoded by this gene is termed LL-37. The presence of LL-37 was first reported in myeloid cells, and it is also found on body surfaces, such as the skin, respiratory epithelium, and ocular surface [8-12]. The antimicrobial activities of LL-37 have been reported [13], including a statistically significant inhibitory effect in reducing viral titer of HAdV type 19 (HAdV19) [12]. However, a viral plaque method based on the conventional cytopathic effect (CPE) assay was used in a previous report [12]. In the present study, quantitative polymerase chain reaction (PCR) methods were used to investigate the in vitro anti-adenoviral activity of LL-37 in several adenovirus types capable of inducing keratoconjunctivitis.
A549 cells (alveolar epithelial cells, ATCC no. CCL-185) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Eagle's minimum essential medium (MEM) containing 2 mM L-glutamine, 0.1 mM nonessential amino acids, and 7% fetal calf serum (FCS).
The viruses used were HAdV3, HAdV4, HAdV8, HAdV19a (clinical strain) [16], and HAdV37. HAdV3, 4, 8, and 37 were prototype strains and were provided by ATCC. These strains were propagated in A549 cells and stored at -80Ōäā until use.
The cytotoxicity of the test compound was evaluated in A549 cells. An assay was performed on confluent cell layers seeded in 96-well microplates (Falcon 3072; Becton Dickinson, Lincoln Park, NJ, USA). Dilutions of LL-37 were prepared in Eagle's MEM supplemented with 2% FCS. The medium was then discarded and replaced for 24 hours with medium containing eight concentrations of the test agents: 50, 100, 200, 400, 800, 1600, 3200, and 6400 ┬Ąg/mL. Thereafter, the medium was washed and completely replaced, and after a seven day incubation at 37Ōäā with 5% CO2, the cells in the plates were then subjected to 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS)-based colorimetric assay for determining cell viability, according to the manufacturer's instructions (CellTiter 96 AQueous One Solution Reagent; Promega, Leiden, The Netherlands). A490 values, corrected for the cytotoxicity exerted by LL-37 (as determined in mock-infected cultures), were used to calculate the percent cell viability. The 50% cytotoxic concentration (CC50) was determined as the value causing a 50% destruction of the monolayer cells, as determined by regression analysis.
First, A549 cells were seeded in 96-well plates at 10,000 cells per well and incubated for four or five days until confluency was reached. Then, 50 ┬ĄL HAdV, diluted in medium to obtain a virus input of 5 PFU per well, was added to each well. After 2 hours at 37Ōäā, the virus suspension was aspirated, and the medium was replaced with serial dilutions of the test compounds (200 ┬ĄL per well). The concentration of LL-37 was determined between 1/100 and 1/50 CC50 of LL-37, 0, 55, 110, 220, and 440 ┬Ąg/mL. Mock-treated cultures receiving only the test compound were included in each plate. After seven days of incubation at 37Ōäā, microscopy was performed to score the virus-induced CPE. After removal of the culture supernatant, cells and virus particles were lysed by the addition of 70 ┬ĄL lysis buffer (10 mM Tris-HCl [pH 7.8], 0.5% sodium dodecyl sulfate, 5 mM Na2EDTA, 80 ┬Ąg proteinase K per mL), incubated at 50Ōäā for 1 hour, and then at 65Ōäā for 20 minutes to inactivate proteinase K. After clarification (23,000 xg, 10 minutes), cell extracts were stored at -20Ōäā until real-time PCR was performed. The following real-time PCR assay was conducted according to the previous study by Miura-Ochiai et al. [17]. The number of copies of HAdV DNA was calculated from a standard curve using pAd8hxn. The 50% effective concentration (EC50) values were calculated by extrapolation of the compound concentration at which the number of viral DNA copies at seven days post-infection was 50% compared to the value obtained for the virus control. The lower limit for HAdV detection in this study was ten copies/reaction, as reported elsewhere [17]. Experiments were carried out in triplicate for each type, and the mean value was used for evaluation.
The CC50 of LL-37 was 4,400 ┬Ąg/mL (Fig. 1). Based on this value, an experiment to determine its inhibitory effect on HAdV proliferation was carried out at four concentrations: 10-, 20-, 40-, and 80-fold dilution.
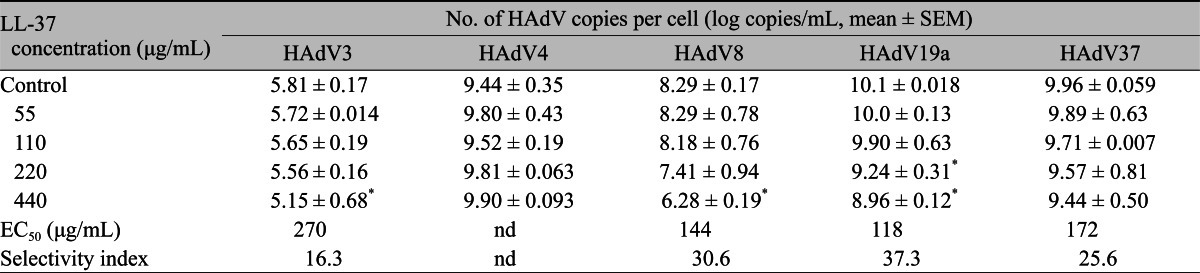
The relationship between LL-37 concentration and the decrease in virus copy is shown in Table 1. LL-37 showed a dose-dependent inhibitory effect on all types, except HAdV4. The EC50 of LL-37 ranged between 118 and 270 ┬Ąg/mL (Table 1). The selectivity index (CC50/EC50) of LL-37 for each HAdV type was calculated based on the values of CC50 and EC50 and ranged from 16.3 to 37.3. In HAdV8 and HAdV19a, LL-37 at 440 ┬Ąg/mL produced a two-log reduction in virus copy compared to the control. A significant correlation between dose and virus reduction was observed in HAdV3, HAdV8, and HAdV19a (Table 1).
Gordon et al. [12] reported that, among the HAdV types tested, HAdV3, 5, 8, and 19 all demonstrated inhibition by LL-37. However, only HAdV19 demonstrated a statistically significant reduction in titer. In the present study, LL-37 showed an inhibitory effect on HAdV3, 8, 19a, and 37, but it was shown that LL-37 was ineffective against HAdV4. From our results, species D seems more sensitive than species B, and species E was resistant to LL-37. The reason for this discrepancy in susceptibility to LL37 among species of HAdV is unclear; however, it might be due to structural differences, especially in the fiber region, which closely relates to the species characteristics and their infectivity [18,19]. There are several other possible explanations. First, the protocol for the antiviral assays used was different in these studies; a time-kill direct inactivation assay based on a conventional CPE assay was used by Gordon et al. [12], whereas a quantitative real-time PCR method, which is a less labor-intensive, faster, and equally reliable way of testing antivirals against HAdV than the CPE assay, was selected in our study. Real-time PCR yielded similar and reproducible antiviral EC50 values [20], and EC50 values were not determined in the previous report [12]. This methodology difference could partly explain the result differences observed. Secondly, the differences in results between the studies may be explained by differences in HAdV strains; clinical strains (wild-type) were used by Gordon et al. [12], whereas all strains except for HAdV19 in the present study were prototype strains that are different from current epidemic strains.
In contrast to HAdV8 and HAdV19 (species D), a statistically significant dose-dependent reduction of virus copy by LL-37 was not observed in HAdV37. The reason for this discrepancy is unclear. As Gordon et al. [12] commented, the differences in the susceptibility of adenovirus types to LL-37 may be related to the known structural differences in the hypervariable region of the hexon capsid and the penton fiber among different HAdV types. Epidemic keratoconjunctivitis is mainly caused by HAdV8, 19, and 37. In contrast to these types, antiviral activity of LL-37 against HAdV-3 is relatively low based on comparison of their EC50 values. Pharyngoconjunctival fever (PCF) is mainly caused by HAdV3 and HAdV4. Thus, LL-37 seems to be not useful as a drug for adenoviral conjunctivitis in PCF. However, there still exists significant dose-dependency for the effect of LL-37 in HAdV3, similar to other types. Thus, further clinical data will be necessary for proper evaluation of LL-37 as a therapeutic measure in the wide spectrum of viral conjunctivitis cases.
When comparing the value of LL-37 (54.0 ┬ĄM) in HAdV19 with those of other agents from our results, values were 1.52 ┬ĄM for zalcitabin [21], 26.1 ┬ĄM for stavudine [21], 52.8 ┬ĄM for N-chlorotaurine [22], and 1,100 ┬ĄM for GRGDSP peptide [23]. From these results, it is assumed that the antiviral effect of LL-37 is weaker than that of conventional antiviral agents, such as zalcitabin and stavudine.
Unlike enveloped viruses, all adenoviridae lack a surrounding host cell-derived lipid membrane, suggesting that direct death through permeabilization of the viral envelope cannot be the operative mechanism [11]. Alternative mechanisms such as disruption of the HAdV particle or blockade of viral entry into the cell might explain the effectiveness of LL-37 against HAdV, as reported in a recent study on defensin [24]. However, we are unable to determine the definite anti-HAdV mechanism of LL-37 at present, and further investigation is required to clarify this issue.
In conclusion, hCAP-18/LL-37, which plays an important role in innate immunity as an antimicrobial peptide, has inhibitory activity against HAdV types belonging to species B and D, which often induce keratoconjunctivitis. These results suggest that hCAP-18/LL-37 may be a possible candidate as an eye drop therapy for adenoviral keratoconjunctivitis.
Acknowledgements
This work was supported by a Grant-in-Aid for Encouragement of Scientists (21592269) from the Ministry of Education, Science, Sports, and Culture of Japan. We thank Dr. W. Gray for editing this manuscript.
REFERENCES
1. De Jong JC, Wermenbol AG, Verweij-Uijterwaal MW, et al. Adenoviruses from human immunodeficiency virus-infected individuals, including two strains that represent new candidate serotypes Ad50 and Ad51 of species B1 and D, respectively. J Clin Microbiol 1999;37:3940-3945.



2. Walsh MP, Seto J, Jones MS, et al. Computational analysis identifies human adenovirus type 55 as a re-emergent acute respiratory disease pathogen. J Clin Microbiol 2010;48:991-993.


3. Kaneko H, Aoki K, Ohno S, et al. Complete genome analysis of a novel intertypic recombinant human adenovirus causing epidemic keratoconjunctivitis in Japan. J Clin Microbiol 2011;49:484-490.



4. Kaneko H, Suzutani T, Aoki K, et al. Epidemiological and virological features of epidemic keratoconjunctivitis due to new human adenovirus type 54 in Japan. Br J Ophthalmol 2011;95:32-36.


7. Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett 1995;374:1-5.


8. Huang LC, Petkova TD, Reins RY, et al. Multifunctional roles of human cathelicidin (LL-37) at the ocular surface. Invest Ophthalmol Vis Sci 2006;47:2369-2380.


9. Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci U S A 1998;95:9541-9546.



10. Frohm M, Agerberth B, Ahangari G, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem 1997;272:15258-15263.


11. Gudmundsson GH, Agerberth B, Odeberg J, et al. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem 1996;238:325-332.


12. Gordon YJ, Huang LC, Romanowski EG, et al. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr Eye Res 2005;30:385-394.



13. McDermott AM. The role of antimicrobial peptides at the ocular surface. Ophthalmic Res 2009;41:60-75.


14. Nagaoka I, Hirota S, Niyonsaba F, et al. Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-alpha by blocking the binding of LPS to CD14(+) cells. J Immunol 2001;167:3329-3338.


15. Turner J, Cho Y, Dinh NN, et al. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother 1998;42:2206-2214.



16. Noda M, Otagaki Y, Ikeda Y, et al. Genome types of adenovirus types 19 and 37 isolated from patients with conjunctivitis in Hiroshima City. J Med Virol 1988;26:15-22.


17. Miura-Ochiai R, Shimada Y, Konno T, et al. Quantitative detection and rapid identification of human adenoviruses. J Clin Microbiol 2007;45:958-967.



18. Shayakhmetov DM, Lieber A. Dependence of adenovirus infectivity on length of the fiber shaft domain. J Virol 2000;74:10274-10286.



19. Ishiko H, Shimada Y, Konno T, et al. Novel human adenovirus causing nosocomial epidemic keratoconjunctivitis. J Clin Microbiol 2008;46:2002-2008.



20. Naesens L, Lenaerts L, Andrei G, et al. Antiadenovirus activities of several classes of nucleoside and nucleotide analogues. Antimicrob Agents Chemother 2005;49:1010-1016.



21. Uchio E, Fuchigami A, Kadonosono K, et al. Anti-adenoviral effect of anti-HIV agents in vitro in serotypes inducing keratoconjunctivitis. Graefes Arch Clin Exp Ophthalmol 2007;245:1319-1325.


22. Uchio E, Inoue H, Kadonosono K. Antiadenoviral effects of N-chlorotaurine in vitro confirmed by quantitative polymerase chain reaction methods. Clin Ophthalmol 2010;4:1325-1329.



- TOOLS




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print




