|
 |
| Korean J Ophthalmol > Volume 26(2); 2012 > Article |
Abstract
In a clinical case series, 5 patients with not-resolved central serous choroidoretinopathy (CSC) lasting more than 1 year received one intravitreal bevacizumab injection (IVB, 1.25 mg) injection. All patients underwent a through ophthalmic examination 1 day, 1 week, and 1, 2, and 6 months after the injection. Best corrected visual acuity (BCVA) and central macular thickness were compared before and after treatment by optical coherence tomography. Mean BCVA was improved significantly (p = 0.020) from 0.60 ± 0.25 to 0.50 ± 0.18 and 0.29 ± 0.19 logarithm of minimum angle of resolution at 6 and 18 weeks, respectively. Central macular thickness was also decreased significantly (p = 0.010) from 370 ± 65 to 208 ± 23 µm at 4 months. No recurrence was occurred during follow-up. IVB injection may have beneficial effect in the treatment of refractory CSC.
Central serous chorioretinopathy (CSC) is one of the several chorioretinal disorders characterized by serous detachment of the neurosensory retina and/or the retinal pigment epithelium (RPE). CSC occurs most frequently in middle-aged men. Major symptoms are blurred vision, usually in one eye only and perceived typically by the patient as a dark spot in the center of the visual field with associated micropsia and metamorphopsia. Normal vision often recurs spontaneously within a few months. The condition can be precipitated by psychosocial stress [1].
Resolution of detachment can usually be achieved in 80% to 90% of acute CSC spontaneously within 3 months. In acute stage, observation is usually the first proposed treatment option. In recurrent or persistent detachment, which is often associated with more diffuse RPE change, however, laser photocoagulation of RPE leakage site(s) is also recommended. It can hasten resolution of associated serous macular detachment, shortens the duration of the disease and reduces the recurrence rate. Nonetheless, this treatment modality is not without risk. It may cause permanent scotoma which may enlarge over time with RPE scar expansion as well as choroidal neovascularization (CNV) development [2,3]. There are also a few reports about beneficial effect of photodynamic therapy (PDT) in chronic CSC. However, it is expensive and cases of CNV have been reported following this treatment [4-6].
Anti-vascular endothelial growth factors, through their anti-permeability characteristics and hence leakage reduction, may be useful in the treatment of cases with refractory CSC and may be helpful as an alternative management. Recently, there are a few case reports with limited number of patients evaluating the effect of intravitreal bevacizumab for treatment of refractory CSC [7-9]. Nevertheless, the literature still suffers from lack of sufficient data in this field. Performing another case series, we also tried to assess the effect of single injection of intravitreal bevacizumab for treatment of refractory CSC.
This prospective interventional case series was approved by the review board/ethics committee of the Ophthalmic Research Center of the university. An informed consent was obtained from each patient.
Five eyes of 5 patients with diagnosis of refractory CSC (lasting more than 1 year) were included in this study. Diagnosis was made by the history of recurrent blurred vision and metamorphopsia for more than one year, detection of neurosensory detachment in ophthalmoscopy and optical coherence tomography (OCT), and observation of active RPE leakage in flourscein angiography. Exclusion criteria consisted of any accompanying macular disease, severe media haziness which precludes OCT evaluation, and noncompliance. Each participant underwent a thorough ophthalmic examination.
All eyes received a single injection of 0.05 mL (1.25 mg) intravitreal bevacizumab (Avastin; Genentech Inc., South San Francisco, CA, USA [made for F. Haffmann-La Roche Ltd., Basel, Switzerland]) performed by a 30-guage needle through supratemporal quadrant 4 mm from the limbus under sterile condition.
All patients underwent a through ophthalmic examination 1 day, 1 week, and 1, 2, and 6 months after the injection. Best corrected visual acuity (BCVA) of the eyes was checked by a masked optometrist. It was changed to the logarithm of minimum angle of resolution (logMAR) scale for statistical purposes and compared at months 2 and 6 with the baseline values. Central macular thickness (CMT) measured by OCT (3D OCT-1000; Topcon Corporation, Tokyo, Japan) was performed at presentation and repeated 6 months after the intervention. It was measured in a 1-mm circle centered on the fovea by an optician who was masked to the study. The data were analyzed by paired t-test. Statistical level of significance was preset at 0.05.
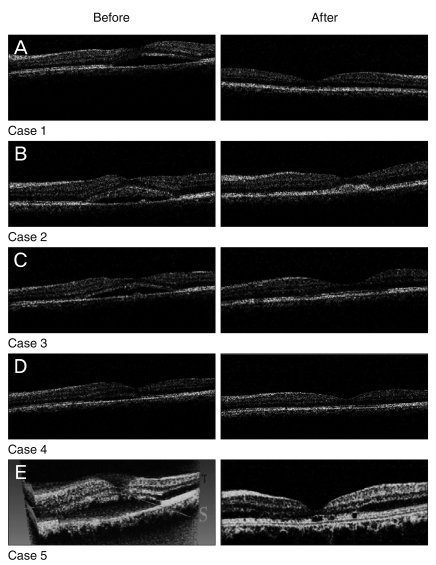
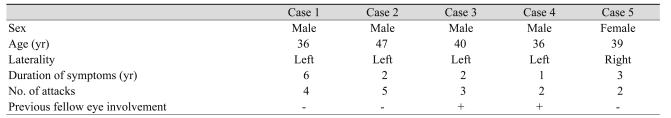
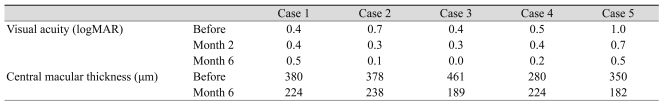
The initial characteristics are listed in Table 1. None of the patients had history of intraocular surgery, diabetes mellitus, hypertension, cardiovascular disease and smoking. An increase of BCVA was noticed during the follow-up in all eyes except case 1. Mean BCVA at baseline was 0.60 ± 0.25 that improved to 0.42 ± 0.16 and 0.24 ± 0.21 logMAR up to the month 2 and 6, respectively. This improvement at 2 months did not reach to a meaningful level (p = 0.064); however, it was statistically significant at 6 months (p = 0.025). No recurrence was observed in any of the eyes during the follow-up period. Central macular thickness decreased significantly from 370 ± 65 µm at baseline to 210 ± 24 µm at 6 months after injections (p = 0.009) (Table 2). Figure 1 demonstrates the OCT pictures of 4 cases before and after treatment. No major ocular or systemic complication was encountered in this study. None of the eyes had intraocular pressure rise (>21 mmHg) or cataract progression during the follow-up period.
Various medications have been suggested for the treatment of CSC by different authors. They include acetazolamide, beta-blockers, vitamins, and non-steroidal anti-inflammatory drugs. None of them has been proved to be beneficial. On the other hand, there are some controversial recommendations in the literature on the use of laser photocoagulation in this field. Some authors reporting that laser photocoagulation shortens the duration of disease and reduces recurrence rate, while others maintain that it does not affect final vision and recurrence rate. Furthermore, laser may be associated with permanent scotoma which may enlarge over time with RPE scar expansion, as the possible development of CNV [2,3].
Photodynamic therapy has also been attempted with some success for treatment of refractory CSC. It may hasten resolution of exudation by reducing choroidal blood flow and hence favoring cessation of leakage [10]. Most recently, several case series have reported the use of indocyanine green guided PDT in the treatment of chronic CSC [11]. Ober et al. [12] reported the successful treatment of focal RPE leaks in CSC by PDT in a small pilot series which showed resolution and visual improvement. Cardillo Piccolino et al. [6] performed indocyanine green guided PDT in 16 eyes with chronic CSC and treatment resulted in complete resolution of serous retinal detachment 1 month after treatment in 75% of eyes. At 3 months after PDT, 69% of eyes had visual improvement of 1 or more lines. However, 31% of their cases developed secondary RPE changes at the site of PDT, which were thought to be due to hypoxic damage caused by choriocapillaris occlusion. Moreover, PDT is an expensive treatment and may cause CNV formation [13].
Our study showed a significant visual improvement and CMT reduction following single injection of IVB (1.25 mg) in 5 cases suffering from refractory CSC for more than one year. In a similar study on 5 cases with CSC, Torres-Soriano et al. noticed an improvement in BCVA, fluorescein angiographic leakage, and reduced or resolved neurosensory detachment. However, they injected 2.5 mg IVB and included cases with history of decreased visual acuity more than 3 months, recurrent episodes of CSC or even acute cases with excessive discomfort about visual acuity [7].
In a case series on 12 eyes, Schaal et al. [9] showed that in cases with chronic CSC IVB injection improved BCVA and reduced CMT. However, they performed multiple injections of 2.5 mg IVB at 6 to 8 week intervals (range, 1 to 4 weeks). However, recurrence did not occur in any case of our study during follow up period.
In summery, the present study demonstrated a promising effect of IVB in the treatment of refractory CSC; however, we can not make specific treatment recommendations based on this small, uncontrolled case series. Further clinical trials with control group are warranted. Further studies with large sample size are warranted.
Notes
This paper was presented as a poster in the AAO/PAAO Joint Meeting, October 2009, San Francisco, CA, USA.
REFERENCES
1. Wang M, Munch IC, Hasler PW, et al. Central serous chorioretinopathy. Acta Ophthalmol 2008;86:126-145.


2. Marmor MF, Tan F. Central serous chorioretinopathy: bilateral multifocal electroretinographic abnormalities. Arch Ophthalmol 1999;117:184-188.


3. Burumcek E, Mudun A, Karacorlu S, Arslan MO. Laser photocoagulation for persistent central serous retinopathy: results of long-term follow-up. Ophthalmology 1997;104:616-622.


4. Spitznas M. Pathogenesis of central serous retinopathy: a new working hypothesis. Graefes Arch Clin Exp Ophthalmol 1986;224:321-324.


5. Marmor MF. New hypotheses on the pathogenesis and treatment of serous retinal detachment. Graefes Arch Clin Exp Ophthalmol 1988;226:548-552.


6. Cardillo Piccolino F, Eandi CM, Ventre L, et al. Photodynamic therapy for chronic central serous chorioretinopathy. Retina 2003;23:752-763.


7. Torres-Soriano ME, Garcia-Aguirre G, Kon-Jara V, et al. A pilot study of intravitreal bevacizumab for the treatment of central serous chorioretinopathy (case reports). Graefes Arch Clin Exp Ophthalmol 2008;246:1235-1239.


8. Von Graefe A. Ueber centrale recidivierende retinitis. Graefes Arch Clin Exp Ophthalmol 1866;12:211-215.
9. Schaal KB, Hoeh AE, Scheuerle A, et al. Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eur J Ophthalmol 2009;19:613-617.


10. Chan WM, Lam DS, Lai TY, et al. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol 2003;87:1453-1458.



11. Battaglia Parodi M, Da Pozzo S, Ravalico G. Photodynamic therapy in chronic central serous chorioretinopathy. Retina 2003;23:235-237.





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print